Targeted AntiBiotics for Chronic pulmonary diseases (TARGET ABC): can targeted antibiotic therapy improve the prognosis of Pseudomonas aeruginosa-infected patients with chronic pulmonary obstructive disease, non-cystic fibrosis bronchiectasis, and asthma? A multicenter, randomized, controlled, open-label trial
- PMID: 36167555
- PMCID: PMC9513970
- DOI: 10.1186/s13063-022-06720-z
Targeted AntiBiotics for Chronic pulmonary diseases (TARGET ABC): can targeted antibiotic therapy improve the prognosis of Pseudomonas aeruginosa-infected patients with chronic pulmonary obstructive disease, non-cystic fibrosis bronchiectasis, and asthma? A multicenter, randomized, controlled, open-label trial
Abstract
Background: Pseudomonas aeruginosa infection is seen in chronic pulmonary disease and is associated with exacerbations and poor long-term prognosis. However, evidence-based guidelines for the management and treatment of P. aeruginosa infection in chronic, non-cystic fibrosis (CF) pulmonary disease are lacking. The aim of this study is to investigate whether targeted antibiotic treatment against P. aeruginosa can reduce exacerbations and mortality in patients with chronic obstructive pulmonary disease (COPD), non-CF bronchiectasis, and asthma.
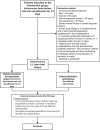
Methods: This study is an ongoing multicenter, randomized, controlled, open-label trial. A total of 150 patients with COPD, non-CF bronchiectasis or asthma, and P. aeruginosa-positive lower respiratory tract samples will be randomly assigned with a 1:1 ratio to either no antibiotic treatment or anti-pseudomonal antibiotic treatment with intravenous beta-lactam and oral ciprofloxacin for 14 days. The primary outcome, analyzed with two co-primary endpoints, is (i) time to prednisolone and/or antibiotic requiring exacerbation or death, in the primary or secondary health sector, within days 20-365 from study allocation and (ii) days alive and without exacerbation within days 20-365 from the study allocation.
Discussion: This trial will determine whether targeted antibiotics can benefit future patients with chronic, non-CF pulmonary disease and P. aeruginosa infection in terms of reduced morbidity and mortality, thus optimizing therapeutic approaches in this large group of chronic patients.
Trial registration: ClinicalTrials.gov NCT03262142 . Registered on August 25, 2017.
Keywords: Antibiotics; Asthma; Chronic obstructive pulmonary disease; Non-CF bronchiectasis; Pseudomonas aeruginosa; Randomized controlled trial.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests. Pradeesh Sivapalan reports personal fees from Boehringer Ingelheim, outside the submitted work.
Figures
References
-
- Soriano JB, Abajobir AA, Abate KH, Abera SF, Agrawal A, Ahmed MB, et al. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2017;5(9):691–706. doi: 10.1016/S2213-2600(17)30293-X. - DOI - PMC - PubMed
-
- GOLD. Global intiative for Chronic Obstructive Lung Disease 2020 Report : pocket guide to COPD daignosis, management, and prevention, a guide for health care professionals. GOLD 2020. 2020. https://goldcopd.org/wpcontent/uploads/2020/03/GOLD-2020-POCKET-GUIDE-ve....
-
- Asthma G initiative for. Global Initiative for Asthma 2019. Glob Strateg Asthma Manag Prev. 2019. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7065541/.