Integrin-specific hydrogels for growth factor-free vasculogenesis
- PMID: 36167724
- PMCID: PMC9515164
- DOI: 10.1038/s41536-022-00253-4
Integrin-specific hydrogels for growth factor-free vasculogenesis
Abstract
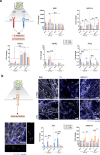
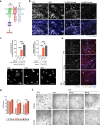
Integrin-binding biomaterials have been extensively evaluated for their capacity to enable de novo formation of capillary-like structures/vessels, ultimately supporting neovascularization in vivo. Yet, the role of integrins as vascular initiators in engineered materials is still not well understood. Here, we show that αvβ3 integrin-specific 3D matrices were able to retain PECAM1+ cells from the stromal vascular fraction (SVF) of adipose tissue, triggering vasculogenesis in vitro in the absence of extrinsic growth factors. Our results suggest that αvβ3-RGD-driven signaling in the formation of capillary-like structures prevents the activation of the caspase 8 pathway and activates the FAK/paxillin pathway, both responsible for endothelial cells (ECs) survival and migration. We also show that prevascularized αvβ3 integrin-specific constructs inosculate with the host vascular system fostering in vivo neovascularization. Overall, this work demonstrates the ability of the biomaterial to trigger vasculogenesis in an integrin-specific manner, by activating essential pathways for EC survival and migration within a self-regulatory growth factor microenvironment. This strategy represents an improvement to current vascularization routes for Tissue Engineering constructs, potentially enhancing their clinical applicability.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Grants and funding
- ERC-2016-COG-726061/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- ERC-2018-STG-805411/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- NORTE-08-5369-FSE-000037/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Euratom (H2020 Euratom Research and Training Programme 2014-2018)
- SFRH/BD/119756/2016/NOVA | Faculdade de Ciências e Tecnologia, Universidade Nova de Lisboa (FCT/UNL)
- PD/BD/135252/2017/NOVA | Faculdade de Ciências e Tecnologia, Universidade Nova de Lisboa (FCT/UNL)
LinkOut - more resources
Full Text Sources
Miscellaneous

