Care cascade of tuberculosis infection treatment for people living with HIV in the era of antiretroviral therapy scale-up
- PMID: 36167744
- PMCID: PMC9515204
- DOI: 10.1038/s41598-022-20394-2
Care cascade of tuberculosis infection treatment for people living with HIV in the era of antiretroviral therapy scale-up
Abstract
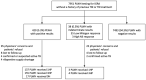
Testing and treatment of tuberculosis infection (TBI) are recommended for people living with HIV (PLWH). We aimed to evaluate the care cascade of TBI treatment among PLWH in the era of antiretroviral therapy (ART) scale-up. This retrospective study included adult PLWH undergoing interferon-gamma release assay (IGRA)-based TBI screening during 2019-2021. PLWH testing IGRA-positive were advised to receive directly-observed therapy for TBI after active TB disease was excluded. The care cascade was evaluated to identify barriers to TBI management. Among 7951 PLWH with a median age of 38 years and CD4 count of 616 cells/mm3, 420 (5.3%) tested positive and 38 (0.5%) indeterminate for IGRA. The TBI treatment initiation rate was 73.6% (309/420) and the completion rate was 91.9% (284/309). More than 80% of PLWH concurrently received short-course rifapentine-based regimens and integrase strand transfer inhibitor (InSTI)-containing ART. The main barrier to treatment initiation was physicians' concerns and patients' refusal (85.6%). The factors associated with treatment non-completion were older age, female, anti-HCV positivity, and higher plasma HIV RNA. Our observation of a high TBI completion rate among PLWH is mainly related to the introduction of short-course rifapentine-based regimens in the InSTI era, which can be the strategy to improve TBI treatment uptake.
© 2022. The Author(s).
Conflict of interest statement
C.C.H. has received research support from Merck, Gilead Sciences, and ViiV and speaker honoraria from Gilead Sciences and ViiV, and served on advisory boards for Gilead Sciences and ViiV. H.Y.S. has received research support from Gilead Sciences. Other authors have no competing interest to disclose.
Figures

References
-
- World Health Organization. Global tuberculosis report 2021. https://www.who.int/publications/i/item/9789240037021 (2021).
-
- World Health Organization. Latent tuberculosis infection: Updated and consolidated guidelines for programmatic management. http://www.who.int/tb/publications/2018/latent-tuberculosis-infection/en/ (2018). - PubMed
-
- Goletti D, Delogu G, Matteelli A, Migliori GB. The role of IGRA in the diagnosis of tuberculosis infection, differentiating from active tuberculosis, and decision making for initiating treatment or preventive therapy of tuberculosis infection. Int. J. Infect. Dis. 2022 doi: 10.1016/j.ijid.2022.02.047. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

