Phospholipid synthesis inside phospholipid membrane vesicles
- PMID: 36167778
- PMCID: PMC9515091
- DOI: 10.1038/s42003-022-03999-1
Phospholipid synthesis inside phospholipid membrane vesicles
Abstract
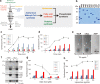
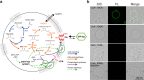
Construction of living artificial cells from genes and molecules can expand our understanding of life system and establish a new aspect of bioengineering. However, growth and division of cell membrane that are basis of cell proliferation are still difficult to reconstruct because a high-yielding phospholipid synthesis system has not been established. Here, we developed a cell-free phospholipid synthesis system that combines fatty acid synthesis and cell-free gene expression system synthesizing acyltransferases. The synthesized fatty acids were sequentially converted into phosphatidic acids by the cell-free synthesized acyltransferases. Because the system can avoid the accumulation of intermediates inhibiting lipid synthesis, sub-millimolar phospholipids could be synthesized within a single reaction mixture. We also performed phospholipid synthesis inside phospholipid membrane vesicles, which encapsulated all the components, and showed the phospholipids localized onto the mother membrane. Our approach would be a platform for the construction of self-reproducing artificial cells since the membrane can grow sustainably.
© 2022. The Author(s).
Conflict of interest statement
Y.K. is applying for a domestic patent (Japan) regarding the technology related to this work. The remaining authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

