Fatty acids homeostasis during fasting predicts protection from chemotherapy toxicity
- PMID: 36167809
- PMCID: PMC9515185
- DOI: 10.1038/s41467-022-33352-3
Fatty acids homeostasis during fasting predicts protection from chemotherapy toxicity
Abstract
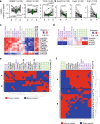
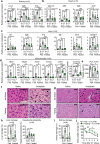
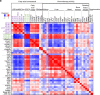
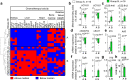
Fasting exerts beneficial effects in mice and humans, including protection from chemotherapy toxicity. To explore the involved mechanisms, we collect blood from humans and mice before and after 36 or 24 hours of fasting, respectively, and measure lipid composition of erythrocyte membranes, circulating micro RNAs (miRNAs), and RNA expression at peripheral blood mononuclear cells (PBMCs). Fasting coordinately affects the proportion of polyunsaturated versus saturated and monounsaturated fatty acids at the erythrocyte membrane; and reduces the expression of insulin signaling-related genes in PBMCs. When fasted for 24 hours before and 24 hours after administration of oxaliplatin or doxorubicin, mice show a strong protection from toxicity in several tissues. Erythrocyte membrane lipids and PBMC gene expression define two separate groups of individuals that accurately predict a differential protection from chemotherapy toxicity, with important clinical implications. Our results reveal a mechanism of fasting associated with lipid homeostasis, and provide biomarkers of fasting to predict fasting-mediated protection from chemotherapy toxicity.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Ruderman NB. Muscle amino acid metabolism and gluconeogenesis. Annu. Rev. Med. 1975;26:245–258. - PubMed
-
- Cahill GF, Owen OE, Morgan AP. The consumption of fuels during prolonged starvation. Adv. Enzym. Regul. 1968;6:143–150. - PubMed
-
- Nuttall FQ, Almokayyad RM, Gannon MC. Comparison of a carbohydrate-free diet vs. fasting on plasma glucose, insulin and glucagon in type 2 diabetes. Metabolism. 2015;64:253–262. - PubMed
-
- Merl V, et al. Serum adiponectin concentrations during a 72-hour fast in over- and normal-weight humans. Int. J. Obes. 2005;29:998–1001. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

