Multianalyte liquid biopsy to aid the diagnostic workup of breast cancer
- PMID: 36167819
- PMCID: PMC9515081
- DOI: 10.1038/s41523-022-00480-4
Multianalyte liquid biopsy to aid the diagnostic workup of breast cancer
Abstract
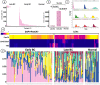
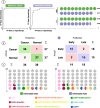
Breast cancer (BC) affects 1 in every 8 women in the United States and is currently the most prevalent cancer worldwide. Precise staging at diagnosis and prognosis are essential components for the clinical management of BC patients. In this study, we set out to evaluate the feasibility of the high-definition single cell (HDSCA) liquid biopsy (LBx) platform to stratify late-stage BC, early-stage BC, and normal donors using peripheral blood samples. Utilizing 5 biomarkers, we identified rare circulating events with epithelial, mesenchymal, endothelial and hematological origin. We detected a higher level of CTCs in late-stage patients, compared to the early-stage and normal donors. Additionally, we observed more tumor-associated large extracellular vesicles (LEVs) in the early-stage, compared to late-stage and the normal donor groups. Overall, we were able to detect reproducible patterns in the enumeration of rare cells and LEVs of cancer vs. normal donors and early-stage vs. late-stage BC with high accuracy, allowing for robust stratification. Our findings illustrate the feasibility of the LBx assay to provide robust detection of rare circulating events in peripheral blood draws and to stratify late-stage BC, early-stage BC, and normal donor samples.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing non-financial interest but the following Financial Competing Interests for Carmen Ruiz Velasco: Inventor receiving royalties on patent licensed to Epic Sciences, Inc.; Anand Kolatkar: receiving stock and royalties, Epic Sciences, Inc., Nicholas Matsumoto: Inventor receiving royalties on patent licensed to Epic Sciences, Inc.; Rafael Nevarez: Inventor receiving royalties on patent licensed to Epic Sciences, Inc.; James B. Hicks: is an unpaid consultant/member on the Clinical Advisory Board, Epic Sciences, Inc.; Peter Kuhn: founder and Chief Scientific Advisor, and received stock and receiving dividends, Epic Sciences, Inc. The rest of the Authors declare no Financial Competing Interests.
Figures




References
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J. Clin. 2021;71:7–33. - PubMed
-
- American Cancer Society, Cancer Facts & Figures 2021, Atlanta: American Cancer Society, (2021).
-
- American Cancer Society. Breast Cancer Facts & Figures 2019-2020, Atlanta: American Cancer Society, Inc., (2019).
-
- Amin MB et al. Cancer Staging Manual, AJCC (American Joint Committee on Cancer), 8th edition, 3rd printing, (2018).
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

