Rejuvenation of the aged brain immune cell landscape in mice through p16-positive senescent cell clearance
- PMID: 36167854
- PMCID: PMC9515187
- DOI: 10.1038/s41467-022-33226-8
Rejuvenation of the aged brain immune cell landscape in mice through p16-positive senescent cell clearance
Abstract
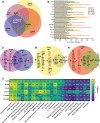
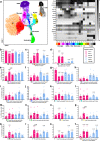
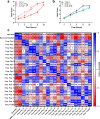
Cellular senescence is a plausible mediator of inflammation-related tissue dysfunction. In the aged brain, senescent cell identities and the mechanisms by which they exert adverse influence are unclear. Here we used high-dimensional molecular profiling, coupled with mechanistic experiments, to study the properties of senescent cells in the aged mouse brain. We show that senescence and inflammatory expression profiles increase with age and are brain region- and sex-specific. p16-positive myeloid cells exhibiting senescent and disease-associated activation signatures, including upregulation of chemoattractant factors, accumulate in the aged mouse brain. Senescent brain myeloid cells promote peripheral immune cell chemotaxis in vitro. Activated resident and infiltrating immune cells increase in the aged brain and are partially restored to youthful levels through p16-positive senescent cell clearance in female p16-InkAttac mice, which is associated with preservation of cognitive function. Our study reveals dynamic remodeling of the brain immune cell landscape in aging and suggests senescent cell targeting as a strategy to counter inflammatory changes and cognitive decline.
© 2022. The Author(s).
Conflict of interest statement
X.Z., T.A.W., E.J.A., J.L.K., T.T., Y.Z., N.K.L., M.J.S., and Mayo Clinic have intellectual property related to this research. This research was reviewed by the Mayo Clinic Conflict of Interest Review Board and was conducted in compliance with Mayo Clinic Conflict of Interest policies. All other authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

