Effectiveness of a structured stimulated spontaneous safety monitoring of medicines reporting program in strengthening pharmacovigilance system in Tanzania
- PMID: 36167960
- PMCID: PMC9515199
- DOI: 10.1038/s41598-022-19884-0
Effectiveness of a structured stimulated spontaneous safety monitoring of medicines reporting program in strengthening pharmacovigilance system in Tanzania
Abstract
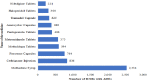
Under-reporting of adverse drug events (ADEs) is a challenge facing developing countries including Tanzania. Given the high magnitude of under-reporting, it was necessary to develop and assess the effectiveness of a 'structured stimulated spontaneous safety monitoring' (SSSSM) reporting program of ADEs which aimed at strengthening pharmacovigilance system in Tanzania. A quasi-experimental design and data mining technique were used to assess the effect of intervention after the introduction of program in seven tertiary hospitals. ADEs reports were collected from a single group and compared for 18 months before (July 2017 to December, 2018) and after the program (January 2019 to June 2020). Out of 16,557 ADEs reports, 98.6% (16,332) were reported after intervention and 0.1% (23) death related to adverse drug reactions (ADRs) were reported. Reports increased from 20 to 11,637 after intervention in Dar es salaam, 49 to 316 in Kilimanjaro and 17 to 77 in Mbeya. The population-based reporting ratio per 1,000,000 inhabitants increased from 2 reports per million inhabitants in 2018 to 85 reports in 2019. The SSSSM program can increase the reporting rate of ADEs and was useful in detecting signals from all types of medicines. This was first effective developed spontaneous program to monitor medicine safety in Tanzania.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Coggins MD. Focus on adverse drug events. Archive. 2016;8:8–10.
-
- Karimi S, Wang C, Metke-Jimenez A, Gaire R, Paris C. Text and data mining techniques in adverse drug reaction detection. ACM Comput. Surv. 2015;47:1–39.
-
- Runciman WB, Roughead EE, Semple SJ, Adams RJ. Adverse drug events and medication errors in Australia. Int. J. Qual. Heal. Care. 2003;15(49):59. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical