The role of the dystrophin glycoprotein complex in muscle cell mechanotransduction
- PMID: 36168044
- PMCID: PMC9515174
- DOI: 10.1038/s42003-022-03980-y
The role of the dystrophin glycoprotein complex in muscle cell mechanotransduction
Abstract
Dystrophin is the central protein of the dystrophin-glycoprotein complex (DGC) in skeletal and heart muscle cells. Dystrophin connects the actin cytoskeleton to the extracellular matrix (ECM). Severing the link between the ECM and the intracellular cytoskeleton has a devastating impact on the homeostasis of skeletal muscle cells, leading to a range of muscular dystrophies. In addition, the loss of a functional DGC leads to progressive dilated cardiomyopathy and premature death. Dystrophin functions as a molecular spring and the DGC plays a critical role in maintaining the integrity of the sarcolemma. Additionally, evidence is accumulating, linking the DGC to mechanosignalling, albeit this role is still less understood. This review article aims at providing an up-to-date perspective on the DGC and its role in mechanotransduction. We first discuss the intricate relationship between muscle cell mechanics and function, before examining the recent research for a role of the dystrophin glycoprotein complex in mechanotransduction and maintaining the biomechanical integrity of muscle cells. Finally, we review the current literature to map out how DGC signalling intersects with mechanical signalling pathways to highlight potential future points of intervention, especially with a focus on cardiomyopathies.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

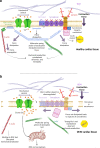
References
-
- Hynes RO. Integrins: A family of cell surface receptors. Cell. 1987;48:549–554. - PubMed
-
- Campbell KP, Kahl SD. Association of dystrophin and an integral membrane glycoprotein. Nature. 1989;338:259–262. - PubMed
-
- Muntoni F, Torelli S, Ferlini A. Dystrophin and mutations: One gene, several proteins, multiple phenotypes. Lancet Neurol. 2003;2:731–740. - PubMed
-
- Guo C, et al. Absence of alpha 7 integrin in dystrophin-deficient mice causes a myopathy similar to Duchenne muscular dystrophy. Hum. Mol. Genet. 2006;15:989–998. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

