Immunotherapy combined with chemotherapy versus chemotherapy alone as the first-line treatment of PD-L1-negative and driver-gene-negative advanced nonsquamous non-small-cell lung cancer: An updated systematic review and meta-analysis
- PMID: 36168110
- PMCID: PMC9663683
- DOI: 10.1111/1759-7714.14664
Immunotherapy combined with chemotherapy versus chemotherapy alone as the first-line treatment of PD-L1-negative and driver-gene-negative advanced nonsquamous non-small-cell lung cancer: An updated systematic review and meta-analysis
Abstract
Background: This meta-analysis aimed to compare the efficacy of immunotherapy combined with chemotherapy versus chemotherapy alone as the first-line therapy for patients with programmed death ligand-1 (PD-L1)-negative and driver-gene-negative advanced nonsquamous non-small-cell lung cancer (NSCLC).
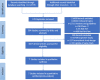
Patients and methods: Eligible randomized trials were identified following the systematic search of PubMed, Cochrane Library, Embase, Web of Science, Wanfang Data, and China Knowledge Resource Integrated Database from January 2000 to June 2022.
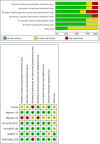
Results: Seven trials involving 1132 patients with PD-L1-negative and driver-gene-negative advanced nonsquamous NSCLC were included. Immunotherapy combined with chemotherapy showed significantly superior objective response rate (ORR) compared with chemotherapy alone (odds ratio 2.81, 95% confidence interval [CI] 1.69-4.65). Immunotherapy combined with chemotherapy also significantly prolonged the progression-free survival (PFS) (hazard ratio [HR] 0.63, 95% CI 0.55-0.74, p < 0.001) and overall survival (OS) (HR 0.68, 95% CI 0.56-0.82, p < 0.001) of patients with PD-L1-negative and driver-gene-negative advanced nonsquamous NSCLC compared to chemotherapy alone. In terms of ≥3 treatment-related adverse events, patients receiving immunotherapy combined with chemotherapy were at higher risk than chemotherapy alone (OR 1.73, 95% CI 1.47-2.05).
Conclusions: This meta-analysis suggested that immunotherapy combined with chemotherapy yielded a better ORR, PFS, and OS, and a higher incidence of treatment-related adverse events as the first-line therapy for patients with PD-L1-negative and driver-gene-negative nonsquamous advanced NSCLC in comparison to chemotherapy alone. A rational treatment protocol should be selected according to the individual condition of the patients.
Keywords: immune checkpoint inhibitor; immunotherapy; meta-analysis; nonsquamous non-small cell lung cancer; programmed death ligand-1.
© 2022 The Authors. Thoracic Cancer published by China Lung Oncology Group and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

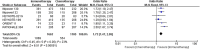
Similar articles
-
Pembrolizumab plus chemotherapy versus chemotherapy alone in patients with advanced non-small cell lung cancer without tumor PD-L1 expression: A pooled analysis of 3 randomized controlled trials.Cancer. 2020 Nov 15;126(22):4867-4877. doi: 10.1002/cncr.33142. Epub 2020 Sep 11. Cancer. 2020. PMID: 32914866 Free PMC article.
-
Efficacy and safety of first-line PD-1/PD-L1 inhibitor in combination with CTLA-4 inhibitor in the treatment of patients with advanced non-small cell lung cancer: a systemic review and meta-analysis.Front Immunol. 2025 Feb 6;16:1515027. doi: 10.3389/fimmu.2025.1515027. eCollection 2025. Front Immunol. 2025. PMID: 39981238 Free PMC article.
-
Association of Survival and Immune-Related Biomarkers With Immunotherapy in Patients With Non-Small Cell Lung Cancer: A Meta-analysis and Individual Patient-Level Analysis.JAMA Netw Open. 2019 Jul 3;2(7):e196879. doi: 10.1001/jamanetworkopen.2019.6879. JAMA Netw Open. 2019. PMID: 31290993 Free PMC article.
-
Safety and Efficacy of PD-1/PD-L1 inhibitors combined with radiotherapy in patients with non-small-cell lung cancer: a systematic review and meta-analysis.Cancer Med. 2021 Feb;10(4):1222-1239. doi: 10.1002/cam4.3718. Epub 2021 Jan 19. Cancer Med. 2021. PMID: 33465302 Free PMC article.
-
Comparative efficacy and safety of PD-1/PD-L1 Inhibitors versus platinum-based chemotherapy for the first-line treatment of advanced non-small cell lung cancer: a meta analysis of randomized controlled trials.Pharmacol Res. 2020 Oct;160:105194. doi: 10.1016/j.phrs.2020.105194. Epub 2020 Sep 13. Pharmacol Res. 2020. PMID: 32937178
Cited by
-
Immune checkpoint inhibitors beyond first-line progression with prior immunotherapy in patients with advanced non-small cell lung cancer.J Thorac Dis. 2023 Apr 28;15(4):1648-1657. doi: 10.21037/jtd-22-1611. Epub 2023 Mar 28. J Thorac Dis. 2023. PMID: 37197488 Free PMC article.
-
Immunotherapy for advanced non-small cell lung cancer with negative programmed death-ligand 1 expression: a literature review.Transl Lung Cancer Res. 2024 Feb 29;13(2):398-422. doi: 10.21037/tlcr-23-144. Epub 2024 Feb 28. Transl Lung Cancer Res. 2024. PMID: 38496691 Free PMC article. Review.
-
Genomic and immunogenomic analysis of three prognostic signature genes in LUAD.BMC Bioinformatics. 2023 Jan 17;24(1):19. doi: 10.1186/s12859-023-05137-y. BMC Bioinformatics. 2023. PMID: 36650426 Free PMC article.
-
Optimal Therapeutic Strategy for PD-L1 Negative Metastatic Non-Small Cell Lung Cancer: A Decision-Making Guide Based on Clinicopathological and Molecular Features.Curr Treat Options Oncol. 2023 Nov;24(11):1550-1567. doi: 10.1007/s11864-023-01132-w. Epub 2023 Oct 6. Curr Treat Options Oncol. 2023. PMID: 37801207 Review.
-
Editorial: Treatment of brain metastases from non-small cell lung cancer: preclinical, clinical, and translational research.Front Oncol. 2025 Apr 4;15:1598159. doi: 10.3389/fonc.2025.1598159. eCollection 2025. Front Oncol. 2025. PMID: 40255424 Free PMC article. No abstract available.
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. - PubMed
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

