ALK‑positive locally advanced lung cancer in a patient who achieved long‑term complete response with crizotinib: A case report
- PMID: 36168410
- PMCID: PMC9475335
- DOI: 10.3892/etm.2022.11587
ALK‑positive locally advanced lung cancer in a patient who achieved long‑term complete response with crizotinib: A case report
Abstract
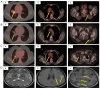
In the last two decades, the existence of key oncogenic alterations, such as activating mutations or chromosomal reorganization, has become crucial in the advanced stage non-small cell lung cancer (NSCLC) treatment paradigm. Among these, anaplastic lymphoma kinase (ALK) gene rearrangement is reported in 3-7% of NSCLC cases worldwide. In patients who respond to long-term ALK therapy, treatment duration is uncertain. The present study reported a case of variant type 1 ALK-rearranged stage 3B lung adenocarcinoma that maintained a complete response for >6 years under treatment with crizotinib. As first-line treatment, crizotinib was administered twice daily (250 mg) and a complete response was confirmed after 3 months. After a complete response to crizotinib for 6 years, the treatment was stopped and the patient was followed up. Multiple brain metastases were detected during the third month of follow-up.
Keywords: anaplastic lymphoma kinase; crizotinib; long-term complete response; lung adenocarcinoma.
Copyright © 2020, Spandidos Publications.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Activity and safety of brigatinib in ALK-rearranged non-small-cell lung cancer and other malignancies: a single-arm, open-label, phase 1/2 trial.Lancet Oncol. 2016 Dec;17(12):1683-1696. doi: 10.1016/S1470-2045(16)30392-8. Epub 2016 Nov 8. Lancet Oncol. 2016. PMID: 27836716 Clinical Trial.
-
Characteristics and Response to Crizotinib in ALK-Rearranged, Advanced Non-Adenocarcinoma, Non-Small Cell Lung Cancer (NA-NSCLC) Patients: a Retrospective Study and Literature Review.Target Oncol. 2018 Oct;13(5):631-639. doi: 10.1007/s11523-018-0592-z. Target Oncol. 2018. PMID: 30218431 Review.
-
Long-term complete response in a patient with postoperative recurrent ALK-rearranged lung adenocarcinoma treated with crizotinib: A case report.Mol Clin Oncol. 2019 Sep;11(3):309-312. doi: 10.3892/mco.2019.1892. Epub 2019 Jul 3. Mol Clin Oncol. 2019. PMID: 31396389 Free PMC article.
-
Crizotinib in patients with advanced, inoperable inflammatory myofibroblastic tumours with and without anaplastic lymphoma kinase gene alterations (European Organisation for Research and Treatment of Cancer 90101 CREATE): a multicentre, single-drug, prospective, non-randomised phase 2 trial.Lancet Respir Med. 2018 Jun;6(6):431-441. doi: 10.1016/S2213-2600(18)30116-4. Epub 2018 Apr 15. Lancet Respir Med. 2018. PMID: 29669701 Clinical Trial.
-
ALK inhibitors in the treatment of advanced NSCLC.Cancer Treat Rev. 2014 Mar;40(2):300-6. doi: 10.1016/j.ctrv.2013.07.002. Epub 2013 Aug 7. Cancer Treat Rev. 2014. PMID: 23931927 Review.
Cited by
-
Refining Criteria for Choosing the First-Line Treatment for Real-World Patients with Advanced ALK-Rearranged NSCLC.Int J Mol Sci. 2025 Jun 21;26(13):5969. doi: 10.3390/ijms26135969. Int J Mol Sci. 2025. PMID: 40649750 Free PMC article. Review.
References
-
- Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, Spitznagel EL, Piccirillo J. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: Analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24:4539–4544. doi: 10.1200/JCO.2005.04.4859. - DOI - PubMed
-
- Barlesi F, Mazieres J, Merlio JP, Debieuvre D, Mosser J, Lena H, Ouafik L, Besse B, Rouquette I, Westeel V, et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: Results of a 1-year nationwide programme of the French cooperative thoracic intergroup (IFCT) Lancet. 2016;387:1415–1426. doi: 10.1016/S0140-6736(16)00004-0. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
