Fossil basicranium clarifies the origin of the avian central nervous system and inner ear
- PMID: 36168759
- PMCID: PMC9515635
- DOI: 10.1098/rspb.2022.1398
Fossil basicranium clarifies the origin of the avian central nervous system and inner ear
Abstract
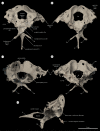
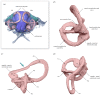
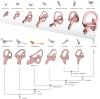
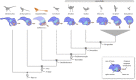
Among terrestrial vertebrates, only crown birds (Neornithes) rival mammals in terms of relative brain size and behavioural complexity. Relatedly, the anatomy of the avian central nervous system and associated sensory structures, such as the vestibular system of the inner ear, are highly modified with respect to those of other extant reptile lineages. However, a dearth of three-dimensional Mesozoic fossils has limited our knowledge of the origins of the distinctive endocranial structures of crown birds. Traits such as an expanded, flexed brain, a ventral connection between the brain and spinal column, and a modified vestibular system have been regarded as exclusive to Neornithes. Here, we demonstrate all of these 'advanced' traits in an undistorted braincase from an Upper Cretaceous enantiornithine bonebed in southeastern Brazil. Our discovery suggests that these crown bird-like endocranial traits may have originated prior to the split between Enantiornithes and the more crownward portion of avian phylogeny over 140 Ma, while coexisting with a remarkably plesiomorphic cranial base and posterior palate region. Altogether, our results support the interpretation that the distinctive endocranial morphologies of crown birds and their Mesozoic relatives are affected by complex trade-offs between spatial constraints during development.
Keywords: birds; brains; dinosaurs; ear; endocranium; labyrinth.
Conflict of interest statement
We declare we have no competing interests.
Figures




References
-
- Romer AS. 1959. Osteology of the Reptiles. Krieger Publishing Company. Malabar, Florida, USA.
-
- Hanken J, Hall BK. 1993. The skull, volume 2: patterns of structural and systematic diversity. Chicago, IL: University of Chicago Press.
-
- O'Connor JK, Chiappe LM. 2011. A revision of enantiornithine (Aves: Ornithothoraces) skull morphology. J. Syst. Paleontol. 9, 135-157. (10.1080/14772019.2010.526639) - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

