The phospholipid flippase ATP8B1 is required for lysosomal fusion in macrophages
- PMID: 36169099
- PMCID: PMC10087937
- DOI: 10.1002/cbf.3752
The phospholipid flippase ATP8B1 is required for lysosomal fusion in macrophages
Abstract
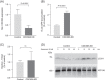
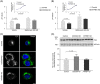
ATP8B1 is a phospholipid flippase and member of the type 4 subfamily of P-type ATPases (P4-ATPase) subfamily. P4-ATPases catalyze the translocation of phospholipids across biological membranes, ensuring proper membrane asymmetry, which is crucial for membrane protein targeting and activity, vesicle biogenesis, and barrier function. Here we have investigated the role of ATP8B1 in the endolysosomal pathway in macrophages. Depletion of ATP8B1 led to delayed degradation of content in the phagocytic pathway and in overacidification of the endolysosomal system. Furthermore, ATP8B1 knockdown cells exhibited large multivesicular bodies filled with intraluminal vesicles. Similar phenotypes were observed in CRISPR-generated ATP8B1 knockout cells. Importantly, induction of autophagy led to accumulation of autophagosomes in ATP8B1 knockdown cells. Collectively, our results support a novel role for ATP8B1 in lysosomal fusion in macrophages, a process crucial in the terminal phase of endolysosomal degradation.
Keywords: ATP8B1; CDC50A; PFIC1; flippase; late endosomes; lysosomal fusion; multivesicular bodies; phospholipid.
© 2022 The Authors. Cell Biochemistry and Function published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

