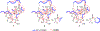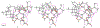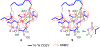Anti-HIV Macrocyclic Daphnane Orthoesters with an Unusual Macrocyclic Ring from Edgeworthia chrysantha
- PMID: 36169204
- PMCID: PMC10114293
- DOI: 10.1021/acs.jnatprod.2c00618
Anti-HIV Macrocyclic Daphnane Orthoesters with an Unusual Macrocyclic Ring from Edgeworthia chrysantha
Abstract
Edgeworthianins A-E (1-5) were isolated from Edgeworthia chrysantha as a class of macrocyclic daphnane orthoesters with an unusual macrocyclic ring formed from a C14 aliphatic chain. Their structures were elucidated by extensive physicochemical and spectroscopic analyses. Compounds 2, 4, and 5 exhibited potent anti-HIV activity against HIV-1 infection of MT4 cells with EC50 values of 29.3, 8.4, and 2.9 nM, respectively. These compounds broaden the findings of the structure-activity relationship of macrocyclic daphnane orthoesters for further anti-HIV drug development.
Figures
References
-
- Kupchan SM; Shizuri Y; Murae T; Swenny JG; Haynes HR; Shen MS; Barrick JC; Bryan AF; vander Helm D; Wu KK J. Am. Chem. Soc 1976, 98, 5719–5720. - PubMed
-
- Liao SG; Chen HD; Yue JM Chem. Rev 2009, 109, 1092–1140. - PubMed
- Hayes PY; Chow S; Somerville MJ; Fletcher MT; De Voss JJ J. Nat. Prod 2010, 73, 1907–1913. - PubMed
- Asada Y; Sukemori A; Watanabe T; Malla KJ; Yoshikawa T; Li W; Koike K; Chen CH; Akiyama T; Qian K; Nakagawa-Goto K; Morris-Natschke SL; Lee KH Org. Lett 2011, 13, 2904–2907. - PMC - PubMed
- Li F; Sun Q; Hong L; Li L; Wu Y; Xia M; Ikejima T; Peng Y; Song S Bioorg. Med. Chem. Lett 2013, 23, 2500–2504. - PubMed
- Yan M; Lu Y; Chen CH; Zhao Y; Lee KH; Chen DF J. Nat. Prod 2015, 78, 2712–2718. - PMC - PubMed
- Guo J; Tian J; Yao G; Zhu H; Xue Y; Luo Z; Zhang J; Zhang Y; Zhang Y Fitoterapia 2015, 106, 242–246. - PubMed
- Li SF; Jiao YY; Zhang ZQ; Chao JB; Jia J; Shi XL; Zhang LW Phytochemistry 2018, 151, 17–25. - PubMed
- Otsuki K; Li W; Asada Y; Chen CH; Lee KH; Koike K Org. Lett 2020, 22, 11–15. - PMC - PubMed
- Otsuki K; Zhang M; Kikuchi T; Tsuji M; Tejima M; Bai ZS; Zhou D; Huang L; Chen CH; Lee KH; Li N; Koike K; Li WJ Nat. Med 2021, 75, 1058–1066. - PubMed
-
- Zayed S; Adolf W; Hafez A; Hecker E Tetrahedron Lett. 1977, 39, 3481–3482.
- Adolf W; Seip EH; Hecker EJ Nat. Prod 1988, 51, 662–674. - PubMed
- He W; Cik M; Van Puyvelde L; Van Dun J; Appendino G; Lesage A; Van der Lindin I; Leysen JE; Wouters W; Mathenge SG; Mudida FP; De Kimpe N 2002, 10, 3245–3255. - PubMed
-
- Huang L; Ho P; Yu J; Zhu L; Lee KH; Chen CH PLoS One 2011, 6, e26677. - PMC - PubMed
- Lai W; Huang L; Zhu L; Ferrari G; Chan C; Li W; Lee KH; Chen CH J. Med. Chem 2015, 58, 8638–8646. - PMC - PubMed
- Huang L; Lai WH; Zhu L; Li W; Wei L; Lee KH; Xie L; Chen CH ACS Med. Chem. Lett 2018, 9, 268–273. - PMC - PubMed
- Liu Q; Cheng YY; Li W; Huang L; Asada Y; Hsieh MT; Morris-Natschke SL; Chen CH; Koike K; K. H. Lee J. Med. Chem 2019, 62, 6958–6971. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical