Camouflage in lichen moths: Field predation experiments and avian vision modelling demonstrate the importance of wing pattern elements and background for survival
- PMID: 36169598
- PMCID: PMC10092008
- DOI: 10.1111/1365-2656.13817
Camouflage in lichen moths: Field predation experiments and avian vision modelling demonstrate the importance of wing pattern elements and background for survival
Abstract
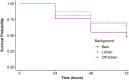
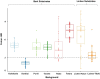
Background matching is perhaps the most ubiquitous form of defensive camouflage in the animal kingdom, an adaptive strategy that relies on the visual resemblance between a prey organism and its background to promote concealment from predators. The importance of background matching has been acknowledged for over a century, yet despite its renown and apparent pervasiveness, few studies exist that have objectively quantified its occurrence and tested the functional significance of background matching in a specific animal study system. The North Island lichen moth Declana atronivea presents a fascinating system to investigate such anti-predator coloration. This species possesses high contrast black and white forewings that appear to resemble lichen. Here we assessed the contribution of background matching to the antipredator defence of D. atronivea using field predation experiments with realistic models. We found that D. atronivea coloration confers a significant survival advantage against native avian predators when on lichen backgrounds compared to bark backgrounds, with an intermediate level of predation occurring when models were near, but not on lichen. This suggests that D. atronivea wing patterns are an adaptation for background matching. We subsequently used calibrated digital photography, avian vision modelling and image analysis techniques to objectively quantify the degree of background matching exhibited by D. atronivea and assessed the contribution of different visual elements (colour, luminance and pattern) to camouflage in this species. Only the pattern elements of D. atronivea presented a close match to that of the lichen backgrounds, with both chromatic and achromatic cues found to be poor predictors of background matching in this species. This study is one of the first to integrate vision modelling, quantitative image analysis and field predation experiments using realistic models to objectively quantify the level and functional significance of background matching in a real species, and presents an ideal system for further investigating the interrelation between multiple mechanisms of camouflage.
Keywords: artificial models; background matching; camouflage; colour analysis; crypsis; lichen resemblance; moth.
© 2022 The Authors. Journal of Animal Ecology published by John Wiley & Sons Ltd on behalf of British Ecological Society.
Conflict of interest statement
The authors have no relevant financial or non‐financial conflict of interest to disclose.
Figures




References
-
- Bates, D. , Mächler, M. , Bolker, B. , & Walker, S. (2015). Fitting linear mixed‐effects models using lme4. Journal of Statistical Software, 67(1), 1–48.
-
- Bond, A. B. , & Kamil, A. C. (2002). Visual predators select for crypticity and polymorphism in virtual prey. Nature, 415(6872), 609–613. - PubMed
-
- Cott, H. B. (1940). Adaptive coloration in animals. Methuen.
-
- Cuthill, I. C. (2019). Camouflage. Journal of Zoology, 308(2), 75–92.
MeSH terms
LinkOut - more resources
Full Text Sources

