Organelles and phytohormones: a network of interactions in plant stress responses
- PMID: 36169618
- PMCID: PMC9675595
- DOI: 10.1093/jxb/erac384
Organelles and phytohormones: a network of interactions in plant stress responses
Abstract
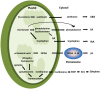
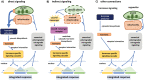
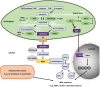
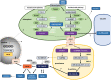
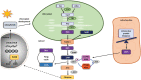
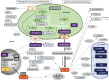
Phytohormones are major signaling components that contribute to nearly all aspects of plant life. They constitute an interconnected communication network to fine-tune growth and development in response to the ever-changing environment. To this end, they have to coordinate with other signaling components, such as reactive oxygen species and calcium signals. On the one hand, the two endosymbiotic organelles, plastids and mitochondria, control various aspects of phytohormone signaling and harbor important steps of hormone precursor biosynthesis. On the other hand, phytohormones have feedback actions on organellar functions. In addition, organelles and phytohormones often act in parallel in a coordinated matter to regulate cellular functions. Therefore, linking organelle functions with increasing knowledge of phytohormone biosynthesis, perception, and signaling will reveal new aspects of plant stress tolerance. In this review, we highlight recent work on organelle-phytohormone interactions focusing on the major stress-related hormones abscisic acid, jasmonates, salicylic acid, and ethylene.
Keywords: Abscisic acid (ABA); chloroplast; ethylene; jasmonates; mitochondria; phytohormones; plant organelles; plastids; retrograde signaling; salicylic acid (SA); stress signaling.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures
Similar articles
-
Phytohormone crosstalk research: cytokinin and its crosstalk with other phytohormones.Curr Protein Pept Sci. 2015;16(5):395-405. doi: 10.2174/1389203716666150330141159. Curr Protein Pept Sci. 2015. PMID: 25824387 Review.
-
Phytohormone signaling and crosstalk in regulating drought stress response in plants.Plant Cell Rep. 2021 Aug;40(8):1305-1329. doi: 10.1007/s00299-021-02683-8. Epub 2021 Mar 22. Plant Cell Rep. 2021. PMID: 33751168 Review.
-
The regulatory role of phytohormones in plant drought tolerance.Planta. 2025 Mar 28;261(5):98. doi: 10.1007/s00425-025-04671-8. Planta. 2025. PMID: 40153011 Review.
-
Abscisic Acid: a versatile phytohormone in plant signaling and beyond.Curr Protein Pept Sci. 2015;16(5):413-34. doi: 10.2174/1389203716666150330130102. Curr Protein Pept Sci. 2015. PMID: 25824385 Review.
-
Phytohormones Regulate Accumulation of Osmolytes Under Abiotic Stress.Biomolecules. 2019 Jul 17;9(7):285. doi: 10.3390/biom9070285. Biomolecules. 2019. PMID: 31319576 Free PMC article. Review.
Cited by
-
The Identification of Transcriptomic and Phytohormonal Biomarkers for Monitoring Drought and Evaluating the Potential of Acibenzolar-S-Methyl Root Application to Prime Two Apple Rootstock Genotypes for Drought Resistance.Int J Mol Sci. 2025 Jul 21;26(14):6986. doi: 10.3390/ijms26146986. Int J Mol Sci. 2025. PMID: 40725234 Free PMC article.
-
Phytohormones as Regulators of Mitochondrial Gene Expression in Arabidopsis thaliana.Int J Mol Sci. 2023 Nov 29;24(23):16924. doi: 10.3390/ijms242316924. Int J Mol Sci. 2023. PMID: 38069246 Free PMC article.
-
Perception of viral infections and initiation of antiviral defence in rice.Nature. 2025 May;641(8061):173-181. doi: 10.1038/s41586-025-08706-8. Epub 2025 Mar 12. Nature. 2025. PMID: 40074903 Free PMC article.
-
The Roles of Phytohormones in Plant Defense Mechanisms Against the Brown Planthopper.Genes (Basel). 2024 Dec 8;15(12):1579. doi: 10.3390/genes15121579. Genes (Basel). 2024. PMID: 39766846 Free PMC article. Review.
-
Integration of multi-omics data and deep phenotyping provides insights into responses to single and combined abiotic stress in potato.Plant Physiol. 2025 Mar 28;197(4):kiaf126. doi: 10.1093/plphys/kiaf126. Plant Physiol. 2025. PMID: 40173380 Free PMC article.
References
-
- Adams S, Grundy J, Veflingstad SR, Dyer NP, Hannah MA, Ott S, Carré IA.. 2018. Circadian control of abscisic acid biosynthesis and signaling pathways revealed by genome-wide analysis of LHY binding targets. New Phytologist 220, 893–907. - PubMed
-
- Arce RC, Carrillo N, Pierella Karlusich JJ.. 2022. The chloroplast redox-responsive transcriptome of Solanaceous plants reveals significant nuclear gene regulatory motifs associated to stress acclimation. Plant Molecular Biology 108, 513–530. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

