Effect of Dimethyl Fumarate vs Interferon β-1a in Patients With Pediatric-Onset Multiple Sclerosis: The CONNECT Randomized Clinical Trial
- PMID: 36169959
- PMCID: PMC9520348
- DOI: 10.1001/jamanetworkopen.2022.30439
Effect of Dimethyl Fumarate vs Interferon β-1a in Patients With Pediatric-Onset Multiple Sclerosis: The CONNECT Randomized Clinical Trial
Abstract
Importance: With few approved multiple sclerosis therapies in the pediatric population, there is a need for further approved treatment options. Limited data exist for dimethyl fumarate (DMF) treatment in pediatric-onset multiple sclerosis (POMS).
Objective: To compare the efficacy, safety, and tolerability of DMF vs intramuscular interferon β-1a (IFNβ-1a) in POMS.
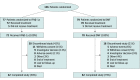
Design, setting, and participants: The CONNECT study was an active-controlled, open-label, rater-blinded 96-week randomized clinical trial in patients with POMS aged 10 to less than 18 years treated between August 2014 and November 2020. Data were analyzed from January through October 2021.
Interventions: Patients were randomized to DMF or IFNβ-1a.
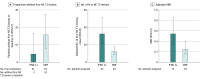
Main outcomes and measures: The primary end point was the proportion of patients free of new or newly enlarging (N or NE) T2 hyperintense lesions at week 96 among trial completers. Secondary end points included number of N or NE T2 lesions, proportion of patients free of relapse, annualized relapse rate (ARR), and safety. The estimated proportion of participants who were relapse free up to week 96 was calculated based on the Kaplan-Meier method. Adjusted ARR was obtained from a negative binomial regression adjusted for baseline relapse rate, baseline Expanded Disability Status Scale (EDSS) score, and age group.
Results: Among 150 patients with POMS in the intention-to-treat (ITT) population (median [range] age, 15 [10-17] years; 101 [67.3%] female patients), 78 individuals received DMF and 72 individuals received IFNβ-1a. At week 96, the proportion of patients with no N or NE T2 hyperintense lesions among 103 trial completers was 16.1% (95% CI, 8.0%-27.7%) for DMF vs 4.9% (95% CI, 0.6%-16.5%) for IFNβ-1a, and in a sensitivity analysis among the ITT population, the proportions were 10 patients receiving DMF (12.8%) vs 2 patients receiving IFNβ-1a (2.8%). The estimated proportion of patients who remained relapse free at week 96 was 66.2% for DMF vs 52.3% for IFNβ-1a. Adjusted ARR (95% CI) at week 96 was 0.24 (95% CI, 0.15-0.39) for DMF vs 0.53 (95% CI, 0.33-0.84) for IFNβ-1a; the rate ratio for DMF vs IFNβ-1a was 0.46 (95% CI, 0.26-0.80; P = .006). The number of treatment-emergent adverse events (TEAEs; 74 patients [94.9%] vs 69 patients [95.8%]), serious TEAEs (18 patients [23.1%] vs 21 patients [29.2%]), and treatment discontinuations due to TEAEs (5 patients [6.4%] vs 8 patients [11.1%]) was similar for DMF vs IFNβ-1a.
Conclusions and relevance: This study found that more pediatric patients with POMS treated with DMF were free of new or newly enlarging T2 lesions and that the adjusted ARR was lower among these patients compared with those treated with interferon β-1a. DMF was well tolerated.
Trial registration: ClinicalTrials.gov Identifier: NCT02283853.
Conflict of interest statement
Figures
Comment in
-
Considering the Future of Pediatric Multiple Sclerosis Trials After the CONNECT Open-Label Randomized Trial.JAMA Netw Open. 2022 Sep 1;5(9):e2230451. doi: 10.1001/jamanetworkopen.2022.30451. JAMA Netw Open. 2022. PMID: 36169961 No abstract available.

