Saliva sample for detection of SARS-CoV-2: A possible alternative for mass testing
- PMID: 36170269
- PMCID: PMC9518879
- DOI: 10.1371/journal.pone.0275201
Saliva sample for detection of SARS-CoV-2: A possible alternative for mass testing
Abstract
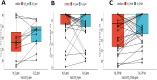
Molecular diagnostic testing has played a critical role in the global response to the novel Coronavirus disease (COVID-19) pandemic, since its first outbreak in late 2019. At the inception of the COVID-19 pandemic, nasopharyngeal swab sample analysis for COVID-19 diagnosis using the real-time polymerase chain reaction (RT-PCR) technique was the most widely used. However, due to the high cost and difficulty of sample collection, the number of available sample types for COVID-19 diagnosis is rapidly increasing, as is the COVID-19 diagnostic literature. The use of nasal swabs, saliva, and oral fluids as viable sample options for the effective detection of SARS-CoV-2 has been implemented successfully in different settings since 2020. These alternative sample type provides a plethora of advantages including decreasing the high exposure risk to frontline workers, enhancing the chances of home self-sampling, reducing the cost, and significantly increasing testing capacity. This study sought to ascertain the effectiveness of Saliva samples as an alternative for COVID-19 diagnosis in Nigeria. Demographic data, paired samples of Nasopharyngeal Swab and Drooling Saliva were obtained from 309 consenting individuals aged 8-83 years presenting for COVID-19 testing. All samples were simultaneously assayed for the detection of SARS-CoV-2 RdRp, N, and E genes using the GeneFinder™ COVID-19 Plus RT-PCR test kit. Out of 309 participants, only 299 with valid RT-PCR results comprising 159 (53.2%) males and 140 (46.8%) females were analyzed in this study using the R Statistical package. Among the 299 samples analyzed, 39 (13.0%) had SARS-CoV-2 detected in at least one specimen type. Both swabs and saliva were positive in 20 (51.3%) participants. Ten participants (25.6%) had swab positive/saliva-negative results and 9 participants (23.1%) had saliva positive/swab-negative results. The percentage of positive and negative agreement of the saliva samples with the nasopharyngeal swab were 67% and 97% respectively with positive and negative predictive values as 69% and 96% respectively. The findings indicate that drooling saliva samples have good and comparable diagnostic accuracy to the nasopharyngeal swabs with moderate sensitivities and high specificities.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Diagnostic Performance of Self-Collected Saliva Versus Nasopharyngeal Swab for the Molecular Detection of SARS-CoV-2 in the Clinical Setting.Microbiol Spectr. 2021 Dec 22;9(3):e0046821. doi: 10.1128/Spectrum.00468-21. Epub 2021 Nov 3. Microbiol Spectr. 2021. PMID: 34730436 Free PMC article.
-
Diagnostic Performance Assessment of Saliva RT-PCR and Nasopharyngeal Antigen for the Detection of SARS-CoV-2 in Peru.Microbiol Spectr. 2022 Aug 31;10(4):e0086122. doi: 10.1128/spectrum.00861-22. Epub 2022 Jul 18. Microbiol Spectr. 2022. PMID: 35867471 Free PMC article.
-
Comparison of SARS-CoV-2 PCR-Based Detection Using Saliva or Nasopharyngeal Swab Specimens in Asymptomatic Populations.Microbiol Spectr. 2021 Sep 3;9(1):e0006221. doi: 10.1128/Spectrum.00062-21. Epub 2021 Aug 25. Microbiol Spectr. 2021. PMID: 34431689 Free PMC article.
-
Diagnostic performance of different sampling approaches for SARS-CoV-2 RT-PCR testing: a systematic review and meta-analysis.Lancet Infect Dis. 2021 Sep;21(9):1233-1245. doi: 10.1016/S1473-3099(21)00146-8. Epub 2021 Apr 12. Lancet Infect Dis. 2021. PMID: 33857405 Free PMC article.
-
The Sensitivity and Costs of Testing for SARS-CoV-2 Infection With Saliva Versus Nasopharyngeal Swabs : A Systematic Review and Meta-analysis.Ann Intern Med. 2021 Apr;174(4):501-510. doi: 10.7326/M20-6569. Epub 2021 Jan 12. Ann Intern Med. 2021. PMID: 33428446 Free PMC article.
Cited by
-
Viruses in saliva from sanctuary chimpanzees (Pan troglodytes) in Republic of Congo and Uganda.PLoS One. 2023 Jun 29;18(6):e0288007. doi: 10.1371/journal.pone.0288007. eCollection 2023. PLoS One. 2023. PMID: 37384730 Free PMC article.
-
Colorimetric RT-LAMP for SARS-CoV-2 detection from nasopharyngeal swabs or crude saliva: a multicountry diagnostic accuracy study in Africa.Lancet Glob Health. 2025 Jul;13(7):e1258-e1267. doi: 10.1016/S2214-109X(25)00150-0. Lancet Glob Health. 2025. PMID: 40580991 Free PMC article.
-
Salivary immune responses after COVID-19 vaccination.PLoS One. 2024 Sep 3;19(9):e0307936. doi: 10.1371/journal.pone.0307936. eCollection 2024. PLoS One. 2024. PMID: 39226256 Free PMC article.
-
SARS-CoV-2, periodontal pathogens, and host factors: The trinity of oral post-acute sequelae of COVID-19.Rev Med Virol. 2024 May;34(3):e2543. doi: 10.1002/rmv.2543. Rev Med Virol. 2024. PMID: 38782605 Free PMC article. Review.
References
-
- World Health Organization (WHO). Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19), 2020. https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mis.... Accessed on 27 August 2020.
-
- Chan JF, Yip CC, To KK, Tang TH, Wong SC, Leung KH, et al.. Improved Molecular Diagnosis of COVID-19 by the Novel, Highly Sensitive and Specific COVID-19-RdRp/Hel Real-Time Reverse Transcription-PCR Assay Validated In Vitro and with Clinical Specimens. J Clin Microbiol. 2020. Apr 23;58(5): e00310–20. doi: 10.1128/JCM.00310-20 . - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

