An "inverse" harpoon mechanism
- PMID: 36170355
- PMCID: PMC9519053
- DOI: 10.1126/sciadv.abq8084
An "inverse" harpoon mechanism
Abstract
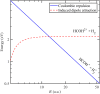
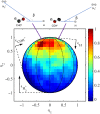
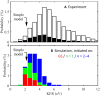
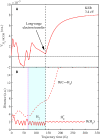
Electron-transfer reactions are ubiquitous in chemistry and biology. The electrons' quantum nature allows their transfer across long distances. For example, in the well-known harpoon mechanism, electron transfer results in Coulombic attraction between initially neutral reactants, leading to a marked increase in the reaction rate. Here, we present a different mechanism in which electron transfer from a neutral reactant to a multiply charged cation results in strong repulsion that encodes the electron-transfer distance in the kinetic energy release. Three-dimensional coincidence imaging allows to identify such "inverse" harpoon products, predicted by nonadiabatic molecular dynamics simulations to occur between H2 and HCOH2+ following double ionization of isolated methanol molecules. These dynamics are experimentally initiated by single-photon double ionization with ultrafast extreme ultraviolet pulses, produced by high-order harmonic generation. A detailed comparison of measured and simulated data indicates that while the relative probability of long-range electron-transfer events is correctly predicted, theory overestimates the electron-transfer distance.
Figures

References
-
- M. Polanyi, Atomic Reactions (Williams and Norgate, 1932, sec. 2, 1932), vol. 3, pp. 28–63.
-
- Blais N. C., Monte Carlo trajectories: The dynamics of harpooning in alkali–halogen reactions. J. Chem. Phys. 49, 9–14 (1968).
-
- Polanyi J. C., Woodall K. B., Energy distribution among reaction products. VI. F+H 2, D 2. J. Chem. Phys. 57, 1574–1586 (1972).
-
- Herschbach D. R., Molecular beam studies of internal excitation of reaction products. Appl. Optics 4, 128 (1965).
-
- Magee J. L., The mechanism of reactions involving excited electronic states the gaseous reactions of the alkali metals and halogens. J. Chem. Phys. 8, 687–698 (1940).
LinkOut - more resources
Full Text Sources

