Sex-specific epigenetic development in the mouse hypothalamic arcuate nucleus pinpoints human genomic regions associated with body mass index
- PMID: 36170368
- PMCID: PMC9519050
- DOI: 10.1126/sciadv.abo3991
Sex-specific epigenetic development in the mouse hypothalamic arcuate nucleus pinpoints human genomic regions associated with body mass index
Abstract
Recent genome-wide association studies corroborate classical research on developmental programming indicating that obesity is primarily a neurodevelopmental disease strongly influenced by nutrition during critical ontogenic windows. Epigenetic mechanisms regulate neurodevelopment; however, little is known about their role in establishing and maintaining the brain's energy balance circuitry. We generated neuron and glia methylomes and transcriptomes from male and female mouse hypothalamic arcuate nucleus, a key site for energy balance regulation, at time points spanning the closure of an established critical window for developmental programming of obesity risk. We find that postnatal epigenetic maturation is markedly cell type and sex specific and occurs in genomic regions enriched for heritability of body mass index in humans. Our results offer a potential explanation for both the limited ontogenic windows for and sex differences in sensitivity to developmental programming of obesity and provide a rich resource for epigenetic analyses of developmental programming of energy balance.
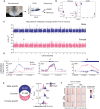
Figures




References
-
- Swinburn B. A., Kraak V. I., Allender S., Atkins V. J., Baker P. I., Bogard J. R., Brinsden H., Calvillo A., De Schutter O., Devarajan R., Ezzati M., Friel S., Goenka S., Hammond R. A., Hastings G., Hawkes C., Herrero M., Hovmand P. S., Howden M., Jaacks L. M., Kapetanaki A. B., Kasman M., Kuhnlein H. V., Kumanyika S. K., Larijani B., Lobstein T., Long M. W., Matsudo V. K. R., Mills S. D. H., Morgan G., Morshed A., Nece P. M., Pan A., Patterson D. W., Sacks G., Shekar M., Simmons G. L., Smit W., Tootee A., Vandevijvere S., Waterlander W. E., Wolfenden L., Dietz W. H., The global syndemic of obesity, undernutrition, and climate change: The Lancet Commission report. Lancet 393, 791–846 (2019). - PubMed
-
- Widdowson E., McCance R. A., The effect of finite periods of undernutrition at different ages on the composition and subsequent development of the rat. Proc. R. Soc. London Ser. B Biol. Sci. 158, 329–342 (1963). - PubMed
-
- Ravelli G. P., Stein Z. A., Susser M. W., Obesity in young men after famine exposure in utero and early infancy. N. Engl. J. Med. 295, 349–353 (1976). - PubMed
-
- Gluckman P. D., Hanson M., Zimmet P., Forrester T., Losing the war against obesity: The need for a developmental perspective. Sci. Transl. Med. 3, 93cm19 (2011). - PubMed

