Gastrointestinal tract drug delivery using algae motors embedded in a degradable capsule
- PMID: 36170380
- PMCID: PMC9884493
- DOI: 10.1126/scirobotics.abo4160
Gastrointestinal tract drug delivery using algae motors embedded in a degradable capsule
Abstract
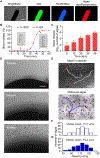
The use of micromotors for active drug delivery via oral administration has recently gained considerable interest. However, efficient motor-assisted delivery into the gastrointestinal (GI) tract remains challenging, owing to the short propulsion lifetime of currently used micromotor platforms. Here, we report on an efficient algae-based motor platform, which takes advantage of the fast and long-lasting swimming behavior of natural microalgae in intestinal fluid to prolong local retention within the GI tract. Fluorescent dye or cell membrane-coated nanoparticle functionalized algae motors were further embedded inside a pH-sensitive capsule to enhance delivery to the small intestines. In vitro, the algae motors displayed a constant motion behavior in simulated intestinal fluid after 12 hours of continuous operation. When orally administered in vivo into mice, the algae motors substantially improved GI distribution of the dye payload compared with traditional magnesium-based micromotors, which are limited by short propulsion lifetimes, and they also enhanced retention of a model chemotherapeutic payload in the GI tract compared with a passive nanoparticle formulation. Overall, combining the efficient motion and extended lifetime of natural algae-based motors with the protective capabilities of oral capsules results in a promising micromotor platform capable of achieving greatly improved cargo delivery in GI tissue for practical biomedical applications.
Conflict of interest statement
Figures






References
-
- Abramson A, Frederiksen MR, Vegge A, Jensen B, Poulsen M, Mouridsen B, Jespersen MO, Kirk RK, Windum J, Hubálek F, Water JJ, Fels J, Gunnarsson SB, Bohr A, Straarup EM, Hvitfeld Ley MW, Lu X, Wainer J, Collins J, Tamang S, Ishida K, Hayward A, Herskind P, Buckley ST, Roxhed N, Langer R, Rahbek U, Traverso G, Oral delivery of systemic monoclonal antibodies, peptides and small molecules using gastric auto-injectors. Nat. Biotechnol 40, 103–109 (2022). - PMC - PubMed
-
- Abramson A, Caffarel-Salvador E, Soares V, Minahan D, Tian RY, Lu X, Dellal D, Gao Y, Kim S, Wainer J, Collins J, Tamang S, Hayward A, Yoshitake T, Lee H-C, Fujimoto J, Fels J, Frederiksen MR, Rahbek U, Roxhed N, Langer R, Traverso G. A luminal unfolding microneedle injector for oral delivery of macromolecules. Nat. Med 25, 1512–1518 (2019). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

