Multicenter, Randomized Trial of a Bionic Pancreas in Type 1 Diabetes
- PMID: 36170500
- PMCID: PMC10028490
- DOI: 10.1056/NEJMoa2205225
Multicenter, Randomized Trial of a Bionic Pancreas in Type 1 Diabetes
Abstract
Background: Currently available semiautomated insulin-delivery systems require individualized insulin regimens for the initialization of therapy and meal doses based on carbohydrate counting for routine operation. In contrast, the bionic pancreas is initialized only on the basis of body weight, makes all dose decisions and delivers insulin autonomously, and uses meal announcements without carbohydrate counting.
Methods: In this 13-week, multicenter, randomized trial, we randomly assigned in a 2:1 ratio persons at least 6 years of age with type 1 diabetes either to receive bionic pancreas treatment with insulin aspart or insulin lispro or to receive standard care (defined as any insulin-delivery method with unblinded, real-time continuous glucose monitoring). The primary outcome was the glycated hemoglobin level at 13 weeks. The key secondary outcome was the percentage of time that the glucose level as assessed by continuous glucose monitoring was below 54 mg per deciliter; the prespecified noninferiority limit for this outcome was 1 percentage point. Safety was also assessed.
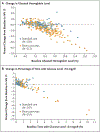
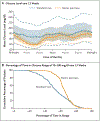
Results: A total of 219 participants 6 to 79 years of age were assigned to the bionic-pancreas group, and 107 to the standard-care group. The glycated hemoglobin level decreased from 7.9% to 7.3% in the bionic-pancreas group and did not change (was at 7.7% at both time points) in the standard-care group (mean adjusted difference at 13 weeks, -0.5 percentage points; 95% confidence interval [CI], -0.6 to -0.3; P<0.001). The percentage of time that the glucose level as assessed by continuous glucose monitoring was below 54 mg per deciliter did not differ significantly between the two groups (13-week adjusted difference, 0.0 percentage points; 95% CI, -0.1 to 0.04; P<0.001 for noninferiority). The rate of severe hypoglycemia was 17.7 events per 100 participant-years in the bionic-pancreas group and 10.8 events per 100 participant-years in the standard-care group (P = 0.39). No episodes of diabetic ketoacidosis occurred in either group.
Conclusions: In this 13-week, randomized trial involving adults and children with type 1 diabetes, use of a bionic pancreas was associated with a greater reduction than standard care in the glycated hemoglobin level. (Funded by the National Institute of Diabetes and Digestive and Kidney Diseases and others; ClinicalTrials.gov number, NCT04200313.).
Copyright © 2022 Massachusetts Medical Society.
Figures
Comment in
-
Seeking Simpler Solutions with Diabetes Technology.N Engl J Med. 2022 Sep 29;387(13):1228-1229. doi: 10.1056/NEJMe2210686. N Engl J Med. 2022. PMID: 36170505 No abstract available.
-
Randomized Trial of a Bionic Pancreas in Type 1 Diabetes.N Engl J Med. 2023 Jan 26;388(4):380. doi: 10.1056/NEJMc2213988. N Engl J Med. 2023. PMID: 36720148 No abstract available.
-
Randomized Trial of a Bionic Pancreas in Type 1 Diabetes.N Engl J Med. 2023 Jan 26;388(4):380. doi: 10.1056/NEJMc2213988. N Engl J Med. 2023. PMID: 36720149 No abstract available.
-
Randomized Trial of a Bionic Pancreas in Type 1 Diabetes. Reply.N Engl J Med. 2023 Jan 26;388(4):380-382. doi: 10.1056/NEJMc2213988. N Engl J Med. 2023. PMID: 36720150 No abstract available.
References
-
- Pettus JH, Zhou FL, Shepherd L, et al. Incidences of severe hypoglycemia and diabetic ketoacidosis and prevalence of microvascular complications stratified by age and glycemic control in U.S. adult patients with type 1 diabetes: a real-world study. Diabetes Care 2019;42:2220–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
