GPRC5D-Targeted CAR T Cells for Myeloma
- PMID: 36170501
- PMCID: PMC10309537
- DOI: 10.1056/NEJMoa2209900
GPRC5D-Targeted CAR T Cells for Myeloma
Abstract
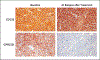
Background: B-cell maturation antigen (BCMA)-directed chimeric antigen receptor (CAR) T-cell therapies have generated responses in patients with advanced myeloma, but relapses are common. G protein-coupled receptor, class C, group 5, member D (GPRC5D) has been identified as an immunotherapeutic target in multiple myeloma. Preclinical studies have shown the efficacy of GPRC5D-targeted CAR T cells, including activity in a BCMA antigen escape model.
Methods: In this phase 1 dose-escalation study, we administered a GPRC5D-targeted CAR T-cell therapy (MCARH109) at four dose levels to patients with heavily pretreated multiple myeloma, including patients with relapse after BCMA CAR T-cell therapy.
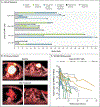
Results: A total of 17 patients were enrolled and received MCARH109 therapy. The maximum tolerated dose was identified at 150×106 CAR T cells. At the 450×106 CAR T-cell dose, 1 patient had grade 4 cytokine release syndrome and immune effector cell-associated neurotoxicity syndrome (ICANS), and 2 patients had a grade 3 cerebellar disorder of unclear cause. No cerebellar disorder, ICANS of any grade, or cytokine release syndrome of grade 3 or higher occurred in the 12 patients who received doses of 25×106 to 150×106 cells. A response was reported in 71% of the patients in the entire cohort and in 58% of those who received doses of 25×106 to 150×106 cells. The patients who had a response included those who had received previous BCMA therapies; responses were observed in 7 of 10 such patients in the entire cohort and in 3 of 6 such patients who received 25×106 to 150×106 cells.
Conclusions: The results of this study of a GPRC5D-targeted CAR T-cell therapy (MCARH109) confirm that GPRC5D is an active immunotherapeutic target in multiple myeloma. (Funded by Juno Therapeutics/Bristol Myers Squibb; ClinicalTrials.gov number, NCT04555551.).
Copyright © 2022 Massachusetts Medical Society.
Figures
Comment in
-
GPRC5D-CAR T cells active in MM.Nat Rev Clin Oncol. 2022 Dec;19(12):747. doi: 10.1038/s41571-022-00701-6. Nat Rev Clin Oncol. 2022. PMID: 36253450 No abstract available.
-
GPRC5D-Targeted CAR T Cells for Myeloma.N Engl J Med. 2022 Dec 15;387(24):2295-2296. doi: 10.1056/NEJMc2213985. N Engl J Med. 2022. PMID: 36516097 No abstract available.
-
GPRC5D-Targeted CAR T Cells for Myeloma. Reply.N Engl J Med. 2022 Dec 15;387(24):2296. doi: 10.1056/NEJMc2213985. N Engl J Med. 2022. PMID: 36516098 No abstract available.
References
-
- Shah UA, Mailankody S. Emerging immunotherapies in multiple myeloma. BMJ 2020; 370:m 3176. - PubMed
-
- Munshi NC, Anderson LD Jr, Shah N, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med 2021; 384: 705–16. - PubMed
-
- Berdeja JG, Madduri D, Usmani SZ, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet 2021; 398: 314–24. - PubMed
-
- Chong EA, Ruella M, Schuster SJ. Five-year outcomes for refractory B-cell lymphomas with CAR T-cell therapy. N Engl J Med 2021; 384: 673–4. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
