Genetically defined individual reference ranges for tryptase limit unnecessary procedures and unmask myeloid neoplasms
- PMID: 36170795
- PMCID: PMC10164828
- DOI: 10.1182/bloodadvances.2022007936
Genetically defined individual reference ranges for tryptase limit unnecessary procedures and unmask myeloid neoplasms
Abstract
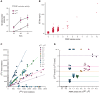
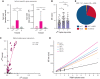
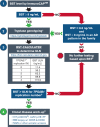
Serum tryptase is a biomarker used to aid in the identification of certain myeloid neoplasms, most notably systemic mastocytosis, where basal serum tryptase (BST) levels >20 ng/mL are a minor criterion for diagnosis. Although clonal myeloid neoplasms are rare, the common cause for elevated BST levels is the genetic trait hereditary α-tryptasemia (HαT) caused by increased germline TPSAB1 copy number. To date, the precise structural variation and mechanism(s) underlying elevated BST in HαT and the general clinical utility of tryptase genotyping, remain undefined. Through cloning, long-read sequencing, and assembling of the human tryptase locus from an individual with HαT, and validating our findings in vitro and in silico, we demonstrate that BST elevations arise from overexpression of replicated TPSAB1 loci encoding canonical α-tryptase protein owing to coinheritance of a linked overactive promoter element. Modeling BST levels based on TPSAB1 replication number, we generate new individualized clinical reference values for the upper limit of normal. Using this personalized laboratory medicine approach, we demonstrate the clinical utility of tryptase genotyping, finding that in the absence of HαT, BST levels >11.4 ng/mL frequently identify indolent clonal mast cell disease. Moreover, substantial BST elevations (eg, >100 ng/mL), which would ordinarily prompt bone marrow biopsy, can result from TPSAB1 replications alone and thus be within normal limits for certain individuals with HαT.
Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution.
Conflict of interest statement
Conflict-of-interest disclosure: Virginia Commonwealth University receives royalties from Thermo Fisher Scientific for their tryptase test that are shared with L.B.S. as its inventor. The remaining authors declare no competing financial interests.
Figures