A nuclear cAMP microdomain suppresses tumor growth by Hippo pathway inactivation
- PMID: 36170819
- PMCID: PMC9549417
- DOI: 10.1016/j.celrep.2022.111412
A nuclear cAMP microdomain suppresses tumor growth by Hippo pathway inactivation
Abstract
Cyclic AMP (cAMP) signaling is localized to multiple spatially distinct microdomains, but the role of cAMP microdomains in cancer cell biology is poorly understood. Here, we present a tunable genetic system that allows us to activate cAMP signaling in specific microdomains. We uncover a nuclear cAMP microdomain that activates a tumor-suppressive pathway in a broad range of cancers by inhibiting YAP, a key effector protein of the Hippo pathway, inside the nucleus. We show that nuclear cAMP induces a LATS-dependent pathway leading to phosphorylation of nuclear YAP solely at serine 397 and export of YAP from the nucleus with no change in YAP protein stability. Thus, nuclear cAMP inhibition of nuclear YAP is distinct from other known mechanisms of Hippo regulation. Pharmacologic targeting of specific cAMP microdomains remains an untapped therapeutic approach for cancer; thus, drugs directed at the nuclear cAMP microdomain may provide avenues for the treatment of cancer.
Keywords: CP: Molecular biology; Hippo pathway; PKA; YAP; cAMP; nucleus; sAC; tumor suppression.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests J.H.Z. is a paid consultant and is on the medical advisory board of Hoth Therapeutics, is on the medical advisory board of SHADE, Inc., and is an inventor on US patent 8859213 on the use of antibodies directed against sACs for the diagnosis of melanocytic proliferations. O.E. is a cofounder and equity holder in Volastra Therapeutics and OneThree Biotech, an equity holder and SAB member in Owkin, Freenome, Genetic Intelligence, and Acuamark DX, and receives funding from Eli Lilly, Janssen, and Sanofi. T.M. is consultant for Leap Therapeutics, Immunos Therapeutics, and Pfizer, is a cofounder of Imvaq Therapeutics, has equity in Imvaq Therapeutics, reports grants from Bristol-Myers Squibb, Surface Oncology, Kyn Therapeutics, Infinity Pharmaceuticals, Peregrine Pharmeceuticals, Adaptive Biotechnologies, Leap Therapeutics, and Aprea, and is an inventor on patent applications related to work on oncolytic viral therapy, alphavirus-based vaccines, neo-antigen modeling, CD40, GITR, OX40, PD-1, and CTLA-4.
Figures
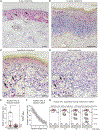
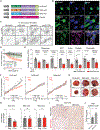

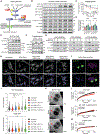

References
-
- Baljinnyam E, De Lorenzo MS, Xie LH, Iwatsubo M, Chen S, Goydos JS, Nowycky MC, and Iwatsubo K (2010). Exchange protein directly activated by cyclic AMP increases melanoma cell migration by a Ca2+-dependent mechanism. Cancer Res. 70, 5607–5617, Epub 2010 Jun 15. 10.1158/0008-5472.can-10-0056. - DOI - PubMed
-
- Barnhill RL, Busam KJ, and Piepkorn MW (2013). Pathology of Melanocytic Nevi and Melanoma (Springer; ). 10.1007/978-3-642-38385-4. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

