Motor cortical influence relies on task-specific activity covariation
- PMID: 36170841
- PMCID: PMC9536049
- DOI: 10.1016/j.celrep.2022.111427
Motor cortical influence relies on task-specific activity covariation
Abstract
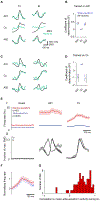
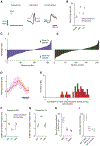
During limb movement, spinal circuits facilitate the alternating activation of antagonistic flexor and extensor muscles. Yet antagonist cocontraction is often required to stabilize joints, like when loads are handled. Previous results suggest that these different muscle activation patterns are mediated by separate flexion- and extension-related motor cortical output populations, while others suggest recruitment of task-specific populations. To distinguish between hypotheses, we developed a paradigm in which mice toggle between forelimb tasks requiring antagonist alternation or cocontraction and measured activity in motor cortical layer 5b. Our results conform to neither hypothesis: consistent flexion- and extension-related activity is not observed across tasks, and no task-specific populations are observed. Instead, activity covariation among motor cortical neurons dramatically changes between tasks, thereby altering the relation between neural and muscle activity. This is also observed specifically for corticospinal neurons. Collectively, our findings indicate that motor cortex drives different muscle activation patterns via task-specific activity covariation.
Keywords: CP: Neuroscience; EMG; alternation; antagonist muscles; caudal forelimb area; cocontraction; motor cortex; mouse; neural activity; neural activity subspace.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





References
-
- Aimonetti JM, and Nielsen JB (2002). Cortical excitability and motor task in man: an investigation of the wrist extensor motor area. Exp. Brain Res 143, 431–439. - PubMed
-
- Akay T, Acharya HJ, Fouad K, and Pearson KG (2006). Behavioral and electromyographic characterization of mice lacking EphA4 receptors. J. Neurophysiol 96, 642–651. - PubMed
-
- Ayling OGS, Harrison TC, Boyd JD, Goroshkov A, and Murphy TH (2009). Automated light-based mapping of motor cortex by photoactivation of channelrhodopsin-2 transgenic mice. Nat. Methods 6, 219–224. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

