Peer Reviewed Evaluation of Registered End-Points of Randomised Trials (the PRE-REPORT study): a stepped wedge, cluster-randomised trial
- PMID: 36171034
- PMCID: PMC9528603
- DOI: 10.1136/bmjopen-2022-066624
Peer Reviewed Evaluation of Registered End-Points of Randomised Trials (the PRE-REPORT study): a stepped wedge, cluster-randomised trial
Abstract
Objective: To test whether providing relevant clinical trial registry information to peer reviewers evaluating trial manuscripts decreases discrepancies between registered and published trial outcomes.
Design: Stepped wedge, cluster-randomised trial, with clusters comprised of eligible manuscripts submitted to each participating journal between 1 November 2018 and 31 October 2019.
Setting: Thirteen medical journals.
Participants: Manuscripts were eligible for inclusion if they were submitted to a participating journal during the study period, presented results from the primary analysis of a clinical trial, and were peer reviewed.
Interventions: During the control phase, there were no changes to pre-existing peer review practices. After journals crossed over into the intervention phase, peer reviewers received a data sheet describing whether trials were registered, the initial registration and enrolment dates, and the registered primary outcome(s) when enrolment began.
Main outcome measure: The presence of a clearly defined, prospectively registered primary outcome consistent with the primary outcome in the published trial manuscript, as determined by two independent outcome assessors.
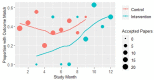
Results: We included 419 manuscripts (243 control and 176 intervention). Participating journals published 43% of control-phase manuscripts and 39% of intervention-phase manuscripts (model-estimated percentage difference between intervention and control trials = -10%, 95% CI -25% to 4%). Among the 173 accepted trials, published primary outcomes were consistent with clearly defined, prospectively registered primary outcomes in 40 of 105 (38%) control-phase trials and 27 of 68 (40%) intervention-phase trials. A linear mixed model did not show evidence of a statistically significant primary outcome effect from the intervention (estimated difference between intervention and control=-6% (90% CI -27% to 15%); one-sided p value=0.68).
Conclusions: These results do not support use of the tested intervention as implemented here to increase agreement between prospectively registered and published trial outcomes. Other approaches are needed to improve the quality of outcome reporting of clinical trials.
Trial registration number: ISRCTN41225307.
Keywords: GENERAL MEDICINE (see Internal Medicine); STATISTICS & RESEARCH METHODS; World Wide Web technology.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at http://www.icmje.org/disclosure-of-interest/ and declare: no support from any commercial organisation for the submitted work; CJ has received research grants from AstraZeneca, Vapotherm, Abbott, and Ophirex outside the submitted work. SS is a full time employee at BMJ but is not involved in editorial decision-making on manuscripts. BM is an employee of the Department of Health and Human Services in the Office of Research Integrity. DLS is an associate editor at JAMA and a deputy editor at Annals of Emergency Medicine. TFP-M is an employee of Ophirex; no other relationships or activities that could appear to have influenced the submitted work.
Figures
Similar articles
-
Reminding Peer Reviewers of Reporting Guideline Items to Improve Completeness in Published Articles: Primary Results of 2 Randomized Trials.JAMA Netw Open. 2023 Jun 1;6(6):e2317651. doi: 10.1001/jamanetworkopen.2023.17651. JAMA Netw Open. 2023. PMID: 37294569 Free PMC article.
-
Peer reviewed evaluation of registered end-points of randomised trials (the PRE-REPORT study): protocol for a stepped-wedge, cluster-randomised trial.BMJ Open. 2019 Jun 1;9(5):e028694. doi: 10.1136/bmjopen-2018-028694. BMJ Open. 2019. PMID: 31154313 Free PMC article.
-
Impact of a short version of the CONSORT checklist for peer reviewers to improve the reporting of randomised controlled trials published in biomedical journals: study protocol for a randomised controlled trial.BMJ Open. 2020 Mar 19;10(3):e035114. doi: 10.1136/bmjopen-2019-035114. BMJ Open. 2020. PMID: 32198306 Free PMC article.
-
Registration and primary outcome reporting in behavioral health trials.BMC Med Res Methodol. 2022 Feb 6;22(1):41. doi: 10.1186/s12874-021-01500-w. BMC Med Res Methodol. 2022. PMID: 35125101 Free PMC article. Review.
-
A Review of COVID-19-Related Publications and Lag Times During the First Six Months of the Year 2020.West J Emerg Med. 2021 Jun 29;22(4):958-962. doi: 10.5811/westjem.2021.3.51737. West J Emerg Med. 2021. PMID: 35354008 Free PMC article. Review.
Cited by
-
There is no reliable evidence that providing authors with customized article templates including items from reporting guidelines improves completeness of reporting: the GoodReports randomized trial (GRReaT).BMC Med Res Methodol. 2025 Mar 14;25(1):71. doi: 10.1186/s12874-025-02518-0. BMC Med Res Methodol. 2025. PMID: 40087548 Free PMC article. Clinical Trial.
-
Methodology reporting improved over time in 176,469 randomized controlled trials.J Clin Epidemiol. 2023 Oct;162:19-28. doi: 10.1016/j.jclinepi.2023.08.004. Epub 2023 Aug 9. J Clin Epidemiol. 2023. PMID: 37562729 Free PMC article.
-
Reminding Peer Reviewers of Reporting Guideline Items to Improve Completeness in Published Articles: Primary Results of 2 Randomized Trials.JAMA Netw Open. 2023 Jun 1;6(6):e2317651. doi: 10.1001/jamanetworkopen.2023.17651. JAMA Netw Open. 2023. PMID: 37294569 Free PMC article.
-
Estimating the prevalence of discrepancies between study registrations and publications: a systematic review and meta-analyses.BMJ Open. 2023 Oct 4;13(10):e076264. doi: 10.1136/bmjopen-2023-076264. BMJ Open. 2023. PMID: 37793922 Free PMC article.
-
Reviewer training for improving grant and journal peer review.Cochrane Database Syst Rev. 2023 Nov 28;11(11):MR000056. doi: 10.1002/14651858.MR000056.pub2. Cochrane Database Syst Rev. 2023. PMID: 38014743 Free PMC article.
References
-
- Assembly WH, World Health Organization . Ministerial Summit on health research: report by the Secretariat, 2005. Available: https://apps.who.int/iris/handle/10665/20304
-
- Regulation (EU) NO 536/2014 of the European Parliament and of the Council of 16 April 2014 on clinical trials on medicinal products for human use, and repealing directive 2001/20/ED. European Union, 2014. Available: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32014R0536
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources