A hygroscopic nano-membrane coating achieves efficient vapor-fed photocatalytic water splitting
- PMID: 36171214
- PMCID: PMC9519874
- DOI: 10.1038/s41467-022-33439-x
A hygroscopic nano-membrane coating achieves efficient vapor-fed photocatalytic water splitting
Abstract
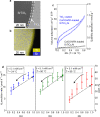
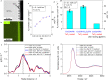
Efficient water vapor splitting opens a new strategy to develop scalable and corrosion-free solar-energy-harvesting systems. This study demonstrates highly efficient overall water splitting under vapor feeding using Al-doped SrTiO3 (SrTiO3:Al)-based photocatalyst decorated homogeneously with nano-membrane TiOx or TaOx thin layers (<3 nm). Here, we show the hygroscopic nature of the metal (hydr)oxide layer provides liquid water reaction environment under vapor, thus achieving an AQY of 54 ± 4%, which is comparable to a liquid reaction. TiOx coated, CoOOH/Rh loaded SrTiO3:Al photocatalyst works for over 100 h, under high pressure (0.3 MPa), and with no problems using simulated seawater as the water vapor supply source. This vapor feeding concept is innovative as a high-pressure-tolerant photoreactor and may have value for large-scale applications. It allows uniform distribution of the water reactant into the reactor system without the potential risk of removing photocatalyst powders and eluting some dissolved ions from the reactor.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Pinaud BA, et al. Technical and economic feasibility of centralized facilities for solar hydrogen production via photocatalysis and photoelectrochemistry. Energy Environ. Sci. 2013;6:1983–2002. doi: 10.1039/c3ee40831k. - DOI
-
- Chen S, Takata T, Domen K. Particulate photocatalysts for overall water splitting. Nat. Rev. Mater. 2017;2:1–17. doi: 10.1038/natrevmats.2017.50. - DOI
LinkOut - more resources
Full Text Sources

