Predicting fertility from sperm motility landscapes
- PMID: 36171267
- PMCID: PMC9519750
- DOI: 10.1038/s42003-022-03954-0
Predicting fertility from sperm motility landscapes
Erratum in
-
Author Correction: Predicting fertility from sperm motility landscapes.Commun Biol. 2022 Oct 13;5(1):1089. doi: 10.1038/s42003-022-04060-x. Commun Biol. 2022. PMID: 36229653 Free PMC article. No abstract available.
Abstract
Understanding the organisational principles of sperm motility has both evolutionary and applied impact. The emergence of computer aided systems in this field came with the promise of automated quantification and classification, potentially improving our understanding of the determinants of reproductive success. Yet, nowadays the relationship between sperm variability and fertility remains unclear. Here, we characterize pig sperm motility using t-SNE, an embedding method adequate to study behavioural variability. T-SNE reveals a hierarchical organization of sperm motility across ejaculates and individuals, enabling accurate fertility predictions by means of Bayesian logistic regression. Our results show that sperm motility features, like high-speed and straight-lined motion, correlate positively with fertility and are more relevant than other sources of variability. We propose the combined use of embedding methods with Bayesian inference frameworks in order to achieve a better understanding of the relationship between fertility and sperm motility in animals, including humans.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interest.
Figures
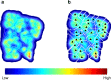
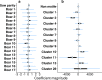


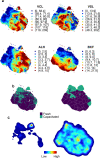

References
-
- Knox RV. Artificial insemination in pigs today. Theriogenology. 2016;85:83–93. - PubMed
-
- Fleming A, et al. Symposium review: the choice and collection of new relevant phenotypes for fertility selection. J. Dairy Sci. 2019;102:3722–3734. - PubMed
-
- Gillan L, Evans G, Maxwell WM. Flow cytometric evaluation of sperm parameters in relation to fertility potential. Theriogenology. 2005;63:445–457. - PubMed
-
- Martínez-Pastor F, Tizado EJ, Garde JJ, Anel L, de Paz P. Statistical series: opportunities and challenges of sperm motility subpopulation analysis. Theriogenology. 2011;75:783–795. - PubMed
-
- Sugihara A, Van Avermaete F, Roelant E, Punjabi U, De Neubourg D. The role of sperm DNA fragmentation testing in predicting intra-uterine insemination outcome: a systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020;244:8–15. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

