PD-1-cis IL-2R agonism yields better effectors from stem-like CD8+ T cells
- PMID: 36171284
- PMCID: PMC9534752
- DOI: 10.1038/s41586-022-05192-0
PD-1-cis IL-2R agonism yields better effectors from stem-like CD8+ T cells
Abstract
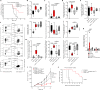
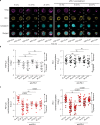
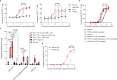
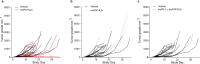
Expansion and differentiation of antigen-experienced PD-1+TCF-1+ stem-like CD8+ T cells into effector cells is critical for the success of immunotherapies based on PD-1 blockade1-4. Hashimoto et al. have shown that, in chronic infections, administration of the cytokine interleukin (IL)-2 triggers an alternative differentiation path of stem-like T cells towards a distinct population of 'better effector' CD8+ T cells similar to those generated in an acute infection5. IL-2 binding to the IL-2 receptor α-chain (CD25) was essential in triggering this alternative differentiation path and expanding better effectors with distinct transcriptional and epigenetic profiles. However, constitutive expression of CD25 on regulatory T cells and some endothelial cells also contributes to unwanted systemic effects from IL-2 therapy. Therefore, engineered IL-2 receptor β- and γ-chain (IL-2Rβγ)-biased agonists are currently being developed6-10. Here we show that IL-2Rβγ-biased agonists are unable to preferentially expand better effector T cells in cancer models and describe PD1-IL2v, a new immunocytokine that overcomes the need for CD25 binding by docking in cis to PD-1. Cis binding of PD1-IL2v to PD-1 and IL-2Rβγ on the same cell recovers the ability to differentiate stem-like CD8+ T cells into better effectors in the absence of CD25 binding in both chronic infection and cancer models and provides superior efficacy. By contrast, PD-1- or PD-L1-blocking antibodies alone, or their combination with clinically relevant doses of non-PD-1-targeted IL2v, cannot expand this unique subset of better effector T cells and instead lead to the accumulation of terminally differentiated, exhausted T cells. These findings provide the basis for the development of a new generation of PD-1 cis-targeted IL-2R agonists with enhanced therapeutic potential for the treatment of cancer and chronic infections.
© 2022. The Author(s).
Conflict of interest statement
Patent application number 15/943,237, with relevance to this work, has been filed by Roche. L.C.D., C.K., L.L., V.N., S.S., I.W. and P.U. are named inventors on this patent family. R.A. holds patents related to the PD-1 pathway. H.K., M.H. and R.A. declare no additional financial interests. L.C.D., V.N., M.K., P.S., L.L., E.M.V., M.R., E.B., J.S., S.J., F.C., M.P., L.K., E.Y., T.H., R.S., M.M., V. Tosevski, S.H., M.B., I.W., S.C., X.G., S.L., A.F.-G., S.S., V. Teichgräber, C.K. and P.U. are employees of Roche and declare ownership of Roche stock. Work performed at Emory for this manuscript was supported by the Roche pRED ROADS program.
Figures










Comment in
-
Interleukin-2 and PD-1 synergistically modify T cell exhaustion programs.Am J Transplant. 2023 Feb;23(2):161-162. doi: 10.1016/j.ajt.2023.01.002. Epub 2023 Jan 14. Am J Transplant. 2023. PMID: 36717354 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

