PD-1 combination therapy with IL-2 modifies CD8+ T cell exhaustion program
- PMID: 36171288
- PMCID: PMC9793890
- DOI: 10.1038/s41586-022-05257-0
PD-1 combination therapy with IL-2 modifies CD8+ T cell exhaustion program
Abstract
Combination therapy with PD-1 blockade and IL-2 is highly effective during chronic lymphocytic choriomeningitis virus infection1. Here we examine the underlying basis for this synergy. We show that PD-1 + IL-2 combination therapy, in contrast to PD-1 monotherapy, substantially changes the differentiation program of the PD-1+TCF1+ stem-like CD8+ T cells and results in the generation of transcriptionally and epigenetically distinct effector CD8+ T cells that resemble highly functional effector CD8+ T cells seen after an acute viral infection. The generation of these qualitatively superior CD8+ T cells that mediate viral control underlies the synergy between PD-1 and IL-2. Our results show that the PD-1+TCF1+ stem-like CD8+ T cells, also referred to as precursors of exhausted CD8+ T cells, are not fate-locked into the exhaustion program and their differentiation trajectory can be changed by IL-2 signals. These virus-specific effector CD8+ T cells emerging from the stem-like CD8+ T cells after combination therapy expressed increased levels of the high-affinity IL-2 trimeric (CD25-CD122-CD132) receptor. This was not seen after PD-1 blockade alone. Finally, we show that CD25 engagement with IL-2 has an important role in the observed synergy between IL-2 cytokine and PD-1 blockade. Either blocking CD25 with an antibody or using a mutated version of IL-2 that does not bind to CD25 but still binds to CD122 and CD132 almost completely abrogated the synergistic effects observed after PD-1 + IL-2 combination therapy. There is considerable interest in PD-1 + IL-2 combination therapy for patients with cancer2,3, and our fundamental studies defining the underlying mechanisms of how IL-2 synergizes with PD-1 blockade should inform these human translational studies.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests
R.A. has patents related to PD-1 pathway (8,652,465 and 9,457,080) licensed to Roche. A.H.S has patents and pending royalties from Roche and Novartis on intellectual property on the PD-1 pathway (patent 7,432,059 with royalties paid from Roche, Merck, Bristol Myers Squibb, EMD-Serono, Boehringer-Ingelheim, AstraZeneca, Leica, Mayo Clinic, Dako and Novartis; patent 7,722,868 with royalties paid from Roche, Merck, Bristol Myers Squibb, EMD-Serono, Boehringer-Ingelheim, AstraZeneca, Leica, Mayo Clinic, Dako and Novartis; patents 8,652,465 and 9,457,080 licensed to Roche; patents 9,683,048, 9,815,898, 9,845,356, 10,202,454 and 10,457,733 licensed to Novartis; and patents 9,580,684, 9,988,452 and 10,370,446 issued to none). G.J.F. has patents and pending royalties on the PD-1-PD-L1 pathway from Roche, Merck MSD, Bristol Myers Squibb, Merck KGaA, Boehringer-Ingelheim, AstraZeneca, Dako, Leica, Mayo Clinic and Novartis (see Supplementary Data 4). G.J.F. has served on advisory boards for Roche, Bristol Myers Squibb, Xios, Origimed, Triursus, iTeos, NextPoint, IgM, Jubilant, Trillium, GV20 and Geode. G.J.F. has equity in Nextpoint, Triursus, Xios, iTeos, IgM, GV20 and Geode. V.T., C.K. and P.U. are employed by Roche with stock options. C.K. and P.U have a patent application with Roche: WO2012107417. The other authors declare no competing interests.
Figures






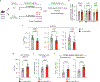






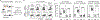





Comment in
-
Interleukin-2 and PD-1 synergistically modify T cell exhaustion programs.Am J Transplant. 2023 Feb;23(2):161-162. doi: 10.1016/j.ajt.2023.01.002. Epub 2023 Jan 14. Am J Transplant. 2023. PMID: 36717354 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI129191/AI/NIAID NIH HHS/United States
- P01 CA236749/CA/NCI NIH HHS/United States
- P01 AI056299/AI/NIAID NIH HHS/United States
- P51 OD011132/OD/NIH HHS/United States
- P50 CA101942/CA/NCI NIH HHS/United States
- U19 AI057266/AI/NIAID NIH HHS/United States
- R00 AI153736/AI/NIAID NIH HHS/United States
- P50 CA217691/CA/NCI NIH HHS/United States
- P30 CA060553/CA/NCI NIH HHS/United States
- P30 CA138292/CA/NCI NIH HHS/United States
- P30 CA016058/CA/NCI NIH HHS/United States
- K99 AI153736/AI/NIAID NIH HHS/United States
- R01 AI030048/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

