Bespoke library docking for 5-HT2A receptor agonists with antidepressant activity
- PMID: 36171289
- PMCID: PMC9996387
- DOI: 10.1038/s41586-022-05258-z
Bespoke library docking for 5-HT2A receptor agonists with antidepressant activity
Abstract
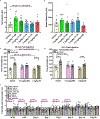
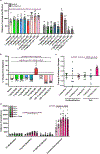
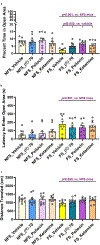
There is considerable interest in screening ultralarge chemical libraries for ligand discovery, both empirically and computationally1-4. Efforts have focused on readily synthesizable molecules, inevitably leaving many chemotypes unexplored. Here we investigate structure-based docking of a bespoke virtual library of tetrahydropyridines-a scaffold that is poorly sampled by a general billion-molecule virtual library but is well suited to many aminergic G-protein-coupled receptors. Using three inputs, each with diverse available derivatives, a one pot C-H alkenylation, electrocyclization and reduction provides the tetrahydropyridine core with up to six sites of derivatization5-7. Docking a virtual library of 75 million tetrahydropyridines against a model of the serotonin 5-HT2A receptor (5-HT2AR) led to the synthesis and testing of 17 initial molecules. Four of these molecules had low-micromolar activities against either the 5-HT2A or the 5-HT2B receptors. Structure-based optimization led to the 5-HT2AR agonists (R)-69 and (R)-70, with half-maximal effective concentration values of 41 nM and 110 nM, respectively, and unusual signalling kinetics that differ from psychedelic 5-HT2AR agonists. Cryo-electron microscopy structural analysis confirmed the predicted binding mode to 5-HT2AR. The favourable physical properties of these new agonists conferred high brain permeability, enabling mouse behavioural assays. Notably, neither had psychedelic activity, in contrast to classic 5-HT2AR agonists, whereas both had potent antidepressant activity in mouse models and had the same efficacy as antidepressants such as fluoxetine at as low as 1/40th of the dose. Prospects for using bespoke virtual libraries to sample pharmacologically relevant chemical space will be considered.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Figures









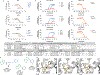


Comment in
-
Virtual screening yields refined GPCR agonists.Nat Rev Drug Discov. 2022 Dec;21(12):879. doi: 10.1038/d41573-022-00177-0. Nat Rev Drug Discov. 2022. PMID: 36284180 No abstract available.
References
References - Methods
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

