State-dependent pupil dilation rapidly shifts visual feature selectivity
- PMID: 36171291
- PMCID: PMC10635574
- DOI: 10.1038/s41586-022-05270-3
State-dependent pupil dilation rapidly shifts visual feature selectivity
Abstract
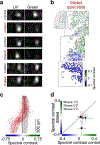
To increase computational flexibility, the processing of sensory inputs changes with behavioural context. In the visual system, active behavioural states characterized by motor activity and pupil dilation1,2 enhance sensory responses, but typically leave the preferred stimuli of neurons unchanged2-9. Here we find that behavioural state also modulates stimulus selectivity in the mouse visual cortex in the context of coloured natural scenes. Using population imaging in behaving mice, pharmacology and deep neural network modelling, we identified a rapid shift in colour selectivity towards ultraviolet stimuli during an active behavioural state. This was exclusively caused by state-dependent pupil dilation, which resulted in a dynamic switch from rod to cone photoreceptors, thereby extending their role beyond night and day vision. The change in tuning facilitated the decoding of ethological stimuli, such as aerial predators against the twilight sky10. For decades, studies in neuroscience and cognitive science have used pupil dilation as an indirect measure of brain state. Our data suggest that, in addition, state-dependent pupil dilation itself tunes visual representations to behavioural demands by differentially recruiting rods and cones on fast timescales.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Figures






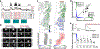
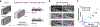


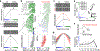



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

