Microdialysis of Drug and Drug Metabolite: a Comprehensive In Vitro Analysis for Voriconazole and Voriconazole N-oxide
- PMID: 36171344
- PMCID: PMC9633485
- DOI: 10.1007/s11095-022-03292-0
Microdialysis of Drug and Drug Metabolite: a Comprehensive In Vitro Analysis for Voriconazole and Voriconazole N-oxide
Abstract
Purpose: Voriconazole is a therapeutically challenging antifungal drug associated with high interindividual pharmacokinetic variability. As a prerequisite to performing clinical trials using the minimally-invasive sampling technique microdialysis, a comprehensive in vitro microdialysis characterization of voriconazole (VRC) and its potentially toxic N-oxide metabolite (NO) was performed.
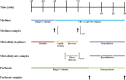
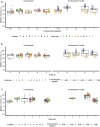
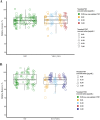
Methods: The feasibility of simultaneous microdialysis of VRC and NO was explored in vitro by investigating the relative recovery (RR) of both compounds in the absence and presence of the other. The dependency of RR on compound combination, concentration, microdialysis catheter and study day was evaluated and quantified by linear mixed-effects modeling.
Results: Median RR of VRC and NO during individual microdialysis were high (87.6% and 91.1%). During simultaneous microdialysis of VRC and NO, median RR did not change (87.9% and 91.1%). The linear mixed-effects model confirmed the absence of significant differences between RR of VRC and NO during individual and simultaneous microdialysis as well as between the two compounds (p > 0.05). No concentration dependency of RR was found (p = 0.284). The study day was the main source of variability (46.3%) while the microdialysis catheter only had a minor effect (4.33%). VRC retrodialysis proved feasible as catheter calibration for both compounds.
Conclusion: These in vitro microdialysis results encourage the application of microdialysis in clinical trials to assess target-site concentrations of VRC and NO. This can support the generation of a coherent understanding of VRC pharmacokinetics and its sources of variability. Ultimately, a better understanding of human VRC pharmacokinetics might contribute to the development of personalized dosing strategies.
Keywords: drug metabolism; exposure; microdialysis; pharmacokinetics; target site; voriconazole.
© 2022. The Author(s).
Figures
Similar articles
-
Microdialysis of Voriconazole and its N-Oxide Metabolite: Amalgamating Knowledge of Distribution and Metabolism Processes in Humans.Pharm Res. 2022 Dec;39(12):3279-3291. doi: 10.1007/s11095-022-03407-7. Epub 2022 Oct 21. Pharm Res. 2022. PMID: 36271205 Free PMC article. Clinical Trial.
-
A versatile high-performance LC-MS/MS assay for the quantification of voriconazole and its N-oxide metabolite in small sample volumes of multiple human matrices for biomedical applications.J Pharm Biomed Anal. 2022 Feb 20;210:114551. doi: 10.1016/j.jpba.2021.114551. Epub 2021 Dec 24. J Pharm Biomed Anal. 2022. PMID: 34999435
-
Contribution of voriconazole N-oxide plasma concentration measurements to voriconazole therapeutic drug monitoring in patients with invasive fungal infection.Mycoses. 2023 May;66(5):396-404. doi: 10.1111/myc.13570. Epub 2023 Feb 5. Mycoses. 2023. PMID: 36698317
-
Voriconazole -- better chances for patients with invasive mycoses.Eur J Med Res. 2002 May 31;7(5):242-56. Eur J Med Res. 2002. PMID: 12069915 Review.
-
Pediatric Clinical Pharmacology of Voriconazole: Role of Pharmacokinetic/Pharmacodynamic Modeling in Pharmacotherapy.Clin Pharmacokinet. 2016 Sep;55(9):1031-43. doi: 10.1007/s40262-016-0379-2. Clin Pharmacokinet. 2016. PMID: 26979736 Review.
Cited by
-
Towards Model-Informed Precision Dosing of Voriconazole: Challenging Published Voriconazole Nonlinear Mixed-Effects Models with Real-World Clinical Data.Clin Pharmacokinet. 2023 Oct;62(10):1461-1477. doi: 10.1007/s40262-023-01274-y. Epub 2023 Aug 21. Clin Pharmacokinet. 2023. PMID: 37603216 Free PMC article.
-
Microdialysis of Voriconazole and its N-Oxide Metabolite: Amalgamating Knowledge of Distribution and Metabolism Processes in Humans.Pharm Res. 2022 Dec;39(12):3279-3291. doi: 10.1007/s11095-022-03407-7. Epub 2022 Oct 21. Pharm Res. 2022. PMID: 36271205 Free PMC article. Clinical Trial.
-
Understanding Voriconazole Metabolism: A Middle-Out Physiologically-Based Pharmacokinetic Modelling Framework Integrating In Vitro and Clinical Insights.Clin Pharmacokinet. 2024 Nov;63(11):1609-1630. doi: 10.1007/s40262-024-01434-8. Epub 2024 Oct 30. Clin Pharmacokinet. 2024. PMID: 39476315 Free PMC article.
References
-
- Brown GD, Denning DW, Gow NAR, Levitz SM, Netea MG, White TC (2012) Hidden Killers: Human Fungal Infections. Science Translational Medicine 4:165rv13–165rv13 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

