Thalamus-driven functional populations in frontal cortex support decision-making
- PMID: 36171427
- PMCID: PMC9534763
- DOI: 10.1038/s41593-022-01171-w
Thalamus-driven functional populations in frontal cortex support decision-making
Abstract
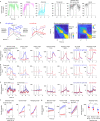
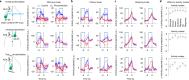
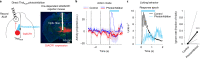
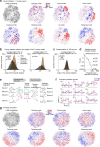
Neurons in frontal cortex exhibit diverse selectivity representing sensory, motor and cognitive variables during decision-making. The neural circuit basis for this complex selectivity remains unclear. We examined activity mediating a tactile decision in mouse anterior lateral motor cortex in relation to the underlying circuits. Contrary to the notion of randomly mixed selectivity, an analysis of 20,000 neurons revealed organized activity coding behavior. Individual neurons exhibited prototypical response profiles that were repeatable across mice. Stimulus, choice and action were coded nonrandomly by distinct neuronal populations that could be delineated by their response profiles. We related distinct selectivity to long-range inputs from somatosensory cortex, contralateral anterior lateral motor cortex and thalamus. Each input connects to all functional populations but with differing strength. Task selectivity was more strongly dependent on thalamic inputs than cortico-cortical inputs. Our results suggest that the thalamus drives subnetworks within frontal cortex coding distinct features of decision-making.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures













Similar articles
-
Maintenance of persistent activity in a frontal thalamocortical loop.Nature. 2017 May 11;545(7653):181-186. doi: 10.1038/nature22324. Epub 2017 May 3. Nature. 2017. PMID: 28467817 Free PMC article.
-
Anterolateral Motor Cortex Connects with a Medial Subdivision of Ventromedial Thalamus through Cell Type-Specific Circuits, Forming an Excitatory Thalamo-Cortico-Thalamic Loop via Layer 1 Apical Tuft Dendrites of Layer 5B Pyramidal Tract Type Neurons.J Neurosci. 2018 Oct 10;38(41):8787-8797. doi: 10.1523/JNEUROSCI.1333-18.2018. Epub 2018 Aug 24. J Neurosci. 2018. PMID: 30143573 Free PMC article.
-
Precise Long-Range Microcircuit-to-Microcircuit Communication Connects the Frontal and Sensory Cortices in the Mammalian Brain.Neuron. 2019 Oct 23;104(2):385-401.e3. doi: 10.1016/j.neuron.2019.06.028. Epub 2019 Jul 29. Neuron. 2019. PMID: 31371111 Free PMC article.
-
Turning Touch into Perception.Neuron. 2020 Jan 8;105(1):16-33. doi: 10.1016/j.neuron.2019.11.033. Neuron. 2020. PMID: 31917952 Review.
-
Functional anatomy of thalamus and basal ganglia.Childs Nerv Syst. 2002 Aug;18(8):386-404. doi: 10.1007/s00381-002-0604-1. Epub 2002 Jul 26. Childs Nerv Syst. 2002. PMID: 12192499 Review.
Cited by
-
Encoding of 2D Self-Centered Plans and World-Centered Positions in the Rat Frontal Orienting Field.J Neurosci. 2024 Sep 11;44(37):e0018242024. doi: 10.1523/JNEUROSCI.0018-24.2024. J Neurosci. 2024. PMID: 39134418 Free PMC article.
-
Behavioral measurements of motor readiness in mice.bioRxiv [Preprint]. 2023 Feb 4:2023.02.03.527054. doi: 10.1101/2023.02.03.527054. bioRxiv. 2023. Update in: Curr Biol. 2023 Sep 11;33(17):3610-3624.e4. doi: 10.1016/j.cub.2023.07.029. PMID: 36778494 Free PMC article. Updated. Preprint.
-
Striatal pathways oppositely shift cortical activity along the decision axis.bioRxiv [Preprint]. 2025 Jul 30:2025.07.29.667406. doi: 10.1101/2025.07.29.667406. bioRxiv. 2025. PMID: 40766374 Free PMC article. Preprint.
-
Separating cognitive and motor processes in the behaving mouse.Nat Neurosci. 2025 Mar;28(3):640-653. doi: 10.1038/s41593-024-01859-1. Epub 2025 Feb 4. Nat Neurosci. 2025. PMID: 39905210
-
Motor learning refines thalamic influence on motor cortex.Nature. 2025 Jul;643(8072):725-734. doi: 10.1038/s41586-025-08962-8. Epub 2025 May 7. Nature. 2025. PMID: 40335698
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

