Awake perception is associated with dedicated neuronal assemblies in the cerebral cortex
- PMID: 36171431
- PMCID: PMC9534770
- DOI: 10.1038/s41593-022-01168-5
Awake perception is associated with dedicated neuronal assemblies in the cerebral cortex
Abstract
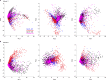
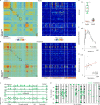
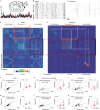
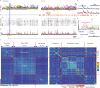
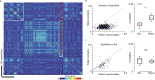
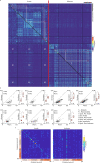
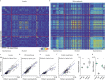
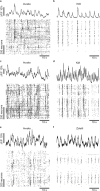
Neural activity in the sensory cortex combines stimulus responses and ongoing activity, but it remains unclear whether these reflect the same underlying dynamics or separate processes. In the present study, we show in mice that, during wakefulness, the neuronal assemblies evoked by sounds in the auditory cortex and thalamus are specific to the stimulus and distinct from the assemblies observed in ongoing activity. By contrast, under three different anesthetics, evoked assemblies are indistinguishable from ongoing assemblies in the cortex. However, they remain distinct in the thalamus. A strong remapping of sensory responses accompanies this dynamic state change produced by anesthesia. Together, these results show that the awake cortex engages dedicated neuronal assemblies in response to sensory inputs, which we suggest is a network correlate of sensory perception.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Sleep Differentially Affects Early and Late Neuronal Responses to Sounds in Auditory and Perirhinal Cortices.J Neurosci. 2020 Apr 1;40(14):2895-2905. doi: 10.1523/JNEUROSCI.1186-19.2020. Epub 2020 Feb 18. J Neurosci. 2020. PMID: 32071140 Free PMC article.
-
Enhanced sound perception by widespread-onset neuronal responses in auditory cortex.Neural Comput. 2007 Dec;19(12):3310-34. doi: 10.1162/neco.2007.19.12.3310. Neural Comput. 2007. PMID: 17970655
-
Microsleep is associated with brain activity patterns unperturbed by auditory inputs.J Neurophysiol. 2019 Dec 1;122(6):2568-2575. doi: 10.1152/jn.00825.2018. Epub 2019 Sep 25. J Neurophysiol. 2019. PMID: 31553690
-
[The auditory system in sleep].Rev Neurol. 2005 Sep 1-15;41(5):280-6. Rev Neurol. 2005. PMID: 16138285 Review. Spanish.
-
Auditory cortex: representation through sparsification?Curr Biol. 2009 Dec 29;19(24):R1123-5. doi: 10.1016/j.cub.2009.11.003. Curr Biol. 2009. PMID: 20064424 Free PMC article. Review.
Cited by
-
Massive perturbation of sound representations by anesthesia in the auditory brainstem.Sci Adv. 2024 Oct 18;10(42):eado2291. doi: 10.1126/sciadv.ado2291. Epub 2024 Oct 18. Sci Adv. 2024. PMID: 39423272 Free PMC article.
-
Anesthesia and the neurobiology of consciousness.Neuron. 2024 May 15;112(10):1553-1567. doi: 10.1016/j.neuron.2024.03.002. Epub 2024 Apr 4. Neuron. 2024. PMID: 38579714 Free PMC article. Review.
-
Safety learning induces postsynaptic potentiation of direct pathway spiny projection neurons in the tail of the striatum.bioRxiv [Preprint]. 2025 May 19:2025.04.29.651089. doi: 10.1101/2025.04.29.651089. bioRxiv. 2025. PMID: 40475679 Free PMC article. Preprint.
-
Bridging functional and anatomical neural connectivity through cluster synchronization.Sci Rep. 2023 Dec 17;13(1):22430. doi: 10.1038/s41598-023-49746-2. Sci Rep. 2023. PMID: 38104227 Free PMC article.
-
Association of bidirectional network cores in the brain with perceptual awareness and cognition.bioRxiv [Preprint]. 2025 Jan 9:2024.04.30.591001. doi: 10.1101/2024.04.30.591001. bioRxiv. 2025. Update in: J Neurosci. 2025 Apr 23;45(17):e0802242025. doi: 10.1523/JNEUROSCI.0802-24.2025. PMID: 38746271 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

