The effect of A1 and A2 reactive astrocyte expression on hydrocephalus shunt failure
- PMID: 36171630
- PMCID: PMC9516791
- DOI: 10.1186/s12987-022-00367-3
The effect of A1 and A2 reactive astrocyte expression on hydrocephalus shunt failure
Abstract
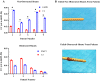
Background: The composition of tissue obstructing neuroprosthetic devices is largely composed of inflammatory cells with a significant astrocyte component. In a first-of-its-kind study, we profile the astrocyte phenotypes present on hydrocephalus shunts.
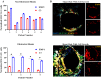
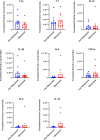
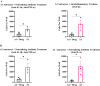
Methods: qPCR and RNA in-situ hybridization were used to quantify pro-inflammatory (A1) and anti-inflammatory (A2) reactive astrocyte phenotypes by analyzing C3 and EMP1 genes, respectively. Additionally, CSF cytokine levels were quantified using ELISA. In an in vitro model of astrocyte growth on shunts, different cytokines were used to prevent the activation of resting astrocytes into the A1 and A2 phenotypes. Obstructed and non-obstructed shunts were characterized based on the degree of actual tissue blockage on the shunt surface instead of clinical diagnosis.
Results: The results showed a heterogeneous population of A1 and A2 reactive astrocytes on the shunts with obstructed shunts having a significantly higher proportion of A2 astrocytes compared to non-obstructed shunts. In addition, the pro-A2 cytokine IL-6 inducing proliferation of astrocytes was found at higher concentrations among CSF from obstructed samples. Consequently, in the in vitro model of astrocyte growth on shunts, cytokine neutralizing antibodies were used to prevent activation of resting astrocytes into the A1 and A2 phenotypes which resulted in a significant reduction in both A1 and A2 growth.
Conclusions: Therefore, targeting cytokines involved with astrocyte A1 and A2 activation is a promising intervention aimed to prevent shunt obstruction.
Keywords: A1 and A2 reactive astrocyte phenotype; Glial Scar; Hydrocephalus; Neuroprosthetic device failure; Targeted drug delivery.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Astrocyte subtype-specific approach to Alzheimer's disease treatment.Neurochem Int. 2021 May;145:104956. doi: 10.1016/j.neuint.2021.104956. Epub 2021 Jan 24. Neurochem Int. 2021. PMID: 33503465 Review.
-
Effect of rottlerin on astrocyte phenotype polarization after trimethyltin insult in the dentate gyrus of mice.J Neuroinflammation. 2022 Jun 11;19(1):142. doi: 10.1186/s12974-022-02507-w. J Neuroinflammation. 2022. PMID: 35690821 Free PMC article.
-
Modulatory effect of IL-1 inhibition following lipopolysaccharide-induced neuroinflammation in neonatal microglia and astrocytes.Int J Dev Neurosci. 2022 May;82(3):243-260. doi: 10.1002/jdn.10179. Epub 2022 Mar 27. Int J Dev Neurosci. 2022. PMID: 35315121
-
A high-resolution real-time quantification of astrocyte cytokine secretion under shear stress for investigating hydrocephalus shunt failure.Commun Biol. 2021 Mar 23;4(1):387. doi: 10.1038/s42003-021-01888-7. Commun Biol. 2021. PMID: 33758339 Free PMC article.
-
Astrocyte polarization in glaucoma: a new opportunity.Neural Regen Res. 2022 Dec;17(12):2582-2588. doi: 10.4103/1673-5374.339470. Neural Regen Res. 2022. PMID: 35662185 Free PMC article. Review.
Cited by
-
TL1A promotes the postoperative cognitive dysfunction in mice through NLRP3-mediated A1 differentiation of astrocytes.CNS Neurosci Ther. 2023 Nov;29(11):3588-3597. doi: 10.1111/cns.14290. Epub 2023 Jun 2. CNS Neurosci Ther. 2023. PMID: 37269079 Free PMC article.
-
MicroRNAs Modulating Neuroinflammation in Parkinson's disease.Inflammation. 2025 Jun;48(3):1042-1062. doi: 10.1007/s10753-024-02125-z. Epub 2024 Aug 20. Inflammation. 2025. PMID: 39162871 Review.
-
Environmental aluminum oxide inducing neurodegeneration in human neurovascular unit with immunity.Sci Rep. 2024 Jan 7;14(1):744. doi: 10.1038/s41598-024-51206-4. Sci Rep. 2024. PMID: 38185738 Free PMC article.
-
Exosomes: a promising microenvironment modulator for spinal cord injury treatment.Int J Biol Sci. 2025 Jun 5;21(8):3791-3824. doi: 10.7150/ijbs.115242. eCollection 2025. Int J Biol Sci. 2025. PMID: 40520019 Free PMC article. Review.
-
Short-Term Effects of Gamma Stimulation on Neuroinflammation at the Tissue-Electrode Interface in Motor Cortex.Neuromodulation. 2024 Apr;27(3):500-508. doi: 10.1016/j.neurom.2023.11.003. Epub 2023 Dec 13. Neuromodulation. 2024. PMID: 38099883 Free PMC article.
References
-
- Karumbaiah L, et al. Relationship between intracortical electrode design and chronic recording function. Biomaterials. 2013;34(33):8061–8074. - PubMed
-
- Shinozaki Y, et al. Transformation of astrocytes to a neuroprotective phenotype by microglia via P2Y1 receptor downregulation. Cell Rep. 2017;19(6):1151–1164. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

