In vitro and in vivo Evaluation of the Bioactive Nanofibers-Encapsulated Benzalkonium Bromide for Accelerating Wound Repair with MRSA Skin Infection
- PMID: 36172005
- PMCID: PMC9510697
- DOI: 10.2147/IJN.S380786
In vitro and in vivo Evaluation of the Bioactive Nanofibers-Encapsulated Benzalkonium Bromide for Accelerating Wound Repair with MRSA Skin Infection
Abstract
Purpose: Developing the ideal drug or dressing is a serious challenge to controlling the occurrence of antibacterial infection during wound healing. Thus, it is important to prepare novel nanofibers for a wound dressing that can control bacterial infections. In our study, the novel self-assembled nanofibers of benzalkonium bromide with bioactive peptide materials of IKVAV and RGD were designed and fabricated.
Methods: Different drug concentration effects of encapsulation efficacy, swelling ratio and strength were determined. Its release profile in simulated wound fluid and its cytotoxicity were studied in vitro. Importantly, the antibacterial efficacy, inhibition of biofilm formation effect and wound healing against MRSA infections in vitro and in vivo were performed after observing the tissue toxicity in vivo.
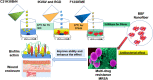
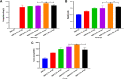
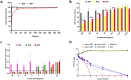
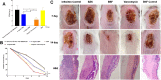
Results: It was found that the optimized drug load (0.8%) was affected by the encapsulation efficacy, swelling ratio, and strength. In addition, the novel nanofibers with average diameter (222.0 nm) and stabile zeta potential (-11.2 mV) have good morphology and characteristics. It has a delayed released profile in the simulated wound fluid and good biocompatibility with L929 cells and most tissues. Importantly, the nanofibers were shown to improve antibacterial efficacy, inhibit biofilm formation, and lead to accelerated wound healing following infection with methicillin-resistant Staphylococcus aureus.
Conclusion: These data suggest that novel nanofibers could effectively shorten the wound-healing time by inhibiting biofilm formation, which make it promising candidates for treatment of MRSA-induced wound infections.
Keywords: antibacterial effect; biofilm; methicillin-resistant Staphylococcus aureus; nanofibers; wound enclosure.
© 2022 Ran et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures




Similar articles
-
Targeting Antibacterial Effect and Promoting of Skin Wound Healing After Infected with Methicillin-Resistant Staphylococcus aureus for the Novel Polyvinyl Alcohol Nanoparticles.Int J Nanomedicine. 2021 Jun 10;16:4031-4044. doi: 10.2147/IJN.S303529. eCollection 2021. Int J Nanomedicine. 2021. PMID: 34140770 Free PMC article.
-
Synergistic berberine chloride and Curcumin-Loaded nanofiber therapies against Methicillin-Resistant Staphylococcus aureus Infection: Augmented immune and inflammatory responses in zebrafish wound healing.Int Immunopharmacol. 2024 Oct 25;140:112856. doi: 10.1016/j.intimp.2024.112856. Epub 2024 Aug 8. Int Immunopharmacol. 2024. PMID: 39121609
-
Honey/Chitosan Nanofiber Wound Dressing Enriched with Allium sativum and Cleome droserifolia: Enhanced Antimicrobial and Wound Healing Activity.ACS Appl Mater Interfaces. 2016 Mar;8(10):6379-90. doi: 10.1021/acsami.6b00739. Epub 2016 Mar 7. ACS Appl Mater Interfaces. 2016. PMID: 26909753
-
Injectable Carrier-Free Hydrogel Dressing with Anti-Multidrug-Resistant Staphylococcus aureus and Anti-Inflammatory Capabilities for Accelerated Wound Healing.ACS Appl Mater Interfaces. 2022 Sep 28;14(38):43035-43049. doi: 10.1021/acsami.2c15463. Epub 2022 Sep 20. ACS Appl Mater Interfaces. 2022. PMID: 36124878
-
Evaluation of a nisin-eluting nanofiber scaffold to treat Staphylococcus aureus-induced skin infections in mice.Antimicrob Agents Chemother. 2013 Aug;57(8):3928-35. doi: 10.1128/AAC.00622-13. Epub 2013 Jun 3. Antimicrob Agents Chemother. 2013. PMID: 23733456 Free PMC article.
Cited by
-
Advances in Electrospun Nanofiber Membranes for Dermatological Applications: A Review.Molecules. 2024 Sep 9;29(17):4271. doi: 10.3390/molecules29174271. Molecules. 2024. PMID: 39275118 Free PMC article. Review.
References
-
- Li M, Liang Y, He J, Zhang H, Guo B. Two-pronged strategy of biomechanically active and biochemically multifunctional hydrogel wound dressing to accelerate wound closure and wound healing. Chem Mater. 2020;32(23):9937–9953. doi:10.1021/acs.chemmater.0c02823 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

