Changing mechanical properties of photopolymerized, dityrosine-crosslinked protein-based hydrogels
- PMID: 36172024
- PMCID: PMC9512244
- DOI: 10.3389/fbioe.2022.1006438
Changing mechanical properties of photopolymerized, dityrosine-crosslinked protein-based hydrogels
Abstract
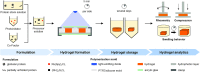
Hydrogels based on renewable resources are a promising class of materials for future applications in pharmaceutics, drug delivery and personalized medicine. Thus, optional adjustments of mechanical properties such as swelling behavior, elasticity and network strength are desired. In this context, hydrogels based on the biological raw materials bovine serum albumin and casein were prepared by dityrosine-crosslinking of their tyrosine residues through visible light-induced photopolymerization. Changing the tyrosine accessibility by urea addition before photopolymerization increased the storage modulus of the hydrogels by 650% while simultaneously being more elastic. Furthermore, contributions of the buffer system composition, variation of protein concentration and storage medium towards mechanical properties of the hydrogel such as storage moduli, elasticity, fracture strain, compressive strength and relative weight swelling ratio are discussed. It could be shown, that changes in precursor solution and storage medium characteristics are crucial parameters towards tuning the mechanical properties of protein-based hydrogels.
Keywords: BSA—bovine serum albumin; casein; protein unfolding; protein-based hydrogels; urea; visible-light induced photopolymerization.
Copyright © 2022 Haas, Körner, Zintel and Hubbuch.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Mechanical Properties of Protein-Based Hydrogels Derived from Binary Protein Mixtures-A Feasibility Study.Polymers (Basel). 2023 Feb 15;15(4):964. doi: 10.3390/polym15040964. Polymers (Basel). 2023. PMID: 36850249 Free PMC article.
-
Genipin-crosslinked casein hydrogels for controlled drug delivery.Int J Pharm. 2009 May 21;373(1-2):41-7. doi: 10.1016/j.ijpharm.2009.02.005. Epub 2009 Feb 12. Int J Pharm. 2009. PMID: 19429286
-
Photopolymerization of cell-encapsulating hydrogels: crosslinking efficiency versus cytotoxicity.Acta Biomater. 2012 May;8(5):1838-48. doi: 10.1016/j.actbio.2011.12.034. Epub 2012 Jan 13. Acta Biomater. 2012. PMID: 22285429
-
Swelling and mechanical properties of physically crosslinked poly(vinyl alcohol) hydrogels.Proc Inst Mech Eng H. 2015 Dec;229(12):828-44. doi: 10.1177/0954411915615469. Proc Inst Mech Eng H. 2015. PMID: 26614797 Review.
-
Processing, mechanical properties and bio-applications of silk fibroin-based high-strength hydrogels.Acta Biomater. 2021 Apr 15;125:57-71. doi: 10.1016/j.actbio.2021.02.018. Epub 2021 Feb 16. Acta Biomater. 2021. PMID: 33601067 Review.
Cited by
-
Investigation of Physicochemical Properties and Surface Morphology of Hydrogel Materials Incorporating Rosehip Extract.Materials (Basel). 2023 Sep 2;16(17):6037. doi: 10.3390/ma16176037. Materials (Basel). 2023. PMID: 37687730 Free PMC article.
-
Milk protein-based hydrogels: Development and biomedical applications.Biomater Transl. 2025 Jun 25;6(2):127-150. doi: 10.12336/bmt.24.00071. eCollection 2025. Biomater Transl. 2025. PMID: 40641996 Free PMC article. Review.
-
Magnetic Resonance Imaging: Time-Dependent Wetting and Swelling Behavior of an Auxetic Hydrogel Based on Natural Polymers.Polymers (Basel). 2022 Nov 19;14(22):5023. doi: 10.3390/polym14225023. Polymers (Basel). 2022. PMID: 36433150 Free PMC article.
-
Mechanical Properties of Protein-Based Hydrogels Derived from Binary Protein Mixtures-A Feasibility Study.Polymers (Basel). 2023 Feb 15;15(4):964. doi: 10.3390/polym15040964. Polymers (Basel). 2023. PMID: 36850249 Free PMC article.
-
Cell biomechanics on muscle atrophy: from intricate mechanisms to therapeutic frontiers.Ann Med. 2025 Dec;57(1):2540598. doi: 10.1080/07853890.2025.2540598. Epub 2025 Aug 1. Ann Med. 2025. PMID: 40747836 Free PMC article. Review.
References
-
- Abaee A., Mohammadian M., Jafari S. M. (2017). Whey and soy protein-based hydrogels and nano-hydrogels as bioactive delivery systems. Trends Food Sci. Technol. 70, 69–81. 10.1016/j.tifs.2017.10.011 - DOI
-
- Alemán J., Chadwick A. V., He J., Hess M., Horie K., Jones R. G., et al. (2007). Definitions of terms relating to the structure and processing of sols, gels, networks, and inorganic-organic hybrid materials (IUPAC recommendations 2007). Pure Appl. Chem. 79, 1801–1829. 10.1351/pac200779101801 - DOI
-
- Almdal K., Dyre J., Hvidt S., Kramer O. (1993). Towards a phenomenological definition of the term “gel. Polym. Gels Netw. 1, 5–17. 10.1016/0966-7822(93)90020-I - DOI
LinkOut - more resources
Full Text Sources

