Anatomical features decide the atypical seizure manifestation of parahypothalamic hamartomas
- PMID: 36172032
- PMCID: PMC9510781
- DOI: 10.3389/fneur.2022.981488
Anatomical features decide the atypical seizure manifestation of parahypothalamic hamartomas
Abstract
Background: The intrahypothalamic phenotype of hypothalamic hamartomas (HH) is associated with epilepsy, and the parahypothalamic phenotype usually leads to central precocious puberty but not neurological comorbidities or seizures. No study has confirmed the pathological role of parahypothalamic hamartomas in epileptogenesis, and the underlying mechanism is yet to be elucidated.
Objective: We aimed to investigate whether parahypothalamic hamartomas are intrinsically epileptogenic and elucidate the underlying pathway of epileptogenesis.
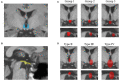
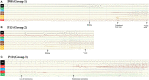
Methods: We reviewed 92 patients with HH-related epilepsy, categorized them by the classification system of Delalande and Fohlen, and further classified Type I (corresponding to parahypothalamic HH) into the following three groups based on the relationship between the lesion and mammillary bodies (MB): entirely invaded (Group 1), partially connected (Group 2), and not connected at all (Group 3). We examined different anatomical features with their relationship to clinical manifestations. Stereoelectroencephalography (SEEG) was implanted in both HH and extra-HH cortices in different groups to identify the epileptogenic zone. Corticocortical evoked potentials (CCEPs) were also used to determine the pathological correlation among different regions to determine the related epileptogenic network.
Results: A total of 13 patients presented with parahypothalamic HH and 10 (76.9%) presented with non-GS only, with late-onset age and normal cognitive development, which is different from classical clinical features. SEEG showed that HH is intrinsically epileptogenic in MB-involved parahypothalamic groups. No statistical difference was found in onset age (p = 0.213), and lesions horizontally oriented from the tuber cinereum without connection to MB were not involved in seizure genesis. CCEP indicated a pathological connection among HH, middle cingulate cortex, and insular cortex.
Conclusion: The parahypothalamic HH can also cause epilepsy and is different from classic HH-related seizures, by non-GS only with the late-onset age and normal cognitive development. MB is proven to be related to non-GS by the mamillo-cingulate-cortex pathway.
Keywords: hypothalamic hamartoma; network; seizure; semiology; stereo-electroencephalography.
Copyright © 2022 Liu, Hu, Zhang, Zheng, Yang, Wang, Mo, Guo, Shao and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Minimally invasive magnetic resonance imaging-guided stereotactic radiofrequency thermocoagulation for epileptogenic hypothalamic hamartomas.Neurosurgery. 2009 Sep;65(3):438-49; discussion 449. doi: 10.1227/01.NEU.0000348292.39252.B5. Neurosurgery. 2009. PMID: 19687687
-
A proposed new classification system of hypothalamic hamartomas in the era of stereotactic ablation surgery.J Neurosurg. 2024 Dec 13;142(4):956-967. doi: 10.3171/2024.7.JNS24560. Print 2025 Apr 1. J Neurosurg. 2024. PMID: 39671576
-
Gelastic seizures and the hypothalamic hamartoma syndrome: Epileptogenesis beyond the lesion?Handb Clin Neurol. 2021;182:143-154. doi: 10.1016/B978-0-12-819973-2.00010-1. Handb Clin Neurol. 2021. PMID: 34266589 Review.
-
Pure endoscopic management of epileptogenic hypothalamic hamartomas.Neurosurg Rev. 2017 Oct;40(4):647-653. doi: 10.1007/s10143-017-0822-3. Epub 2017 Feb 7. Neurosurg Rev. 2017. PMID: 28168619
-
Hypothalamic hamartoma: Epileptogenesis beyond the lesion?Epilepsia. 2017 Jun;58 Suppl 2:32-40. doi: 10.1111/epi.13755. Epilepsia. 2017. PMID: 28591482 Review.
Cited by
-
Epilepsy in hypothalamic hamartomas: semiology spectrum and predictor analyses of 78 patients.Ann Clin Transl Neurol. 2023 Aug;10(8):1365-1373. doi: 10.1002/acn3.51827. Epub 2023 Jun 27. Ann Clin Transl Neurol. 2023. PMID: 37366336 Free PMC article.
-
Adult-onset hypothalamic hamartoma: origin of epilepsy?Acta Epileptol. 2023 Apr 28;5(1):11. doi: 10.1186/s42494-023-00120-9. Acta Epileptol. 2023. PMID: 40217397 Free PMC article.
References
-
- Alomari SO, Houshiemy MNE, Bsat S, Moussalem CK, Allouh M, Omeis IA. Hypothalamic hamartomas: a comprehensive review of the literature - Part 1: Neurobiological features, clinical presentations and advancements in diagnostic tools. Clin Neurol Neurosurg. (2020) 197:106076. 10.1016/j.clineuro.2020.106076 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

