Crystal structure of Arabidopsis DWARF14-LIKE2 (DLK2) reveals a distinct substrate binding pocket architecture
- PMID: 36172078
- PMCID: PMC9470386
- DOI: 10.1002/pld3.446
Crystal structure of Arabidopsis DWARF14-LIKE2 (DLK2) reveals a distinct substrate binding pocket architecture
Abstract
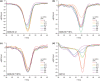
In Arabidopsis thaliana, the Sigma factor B regulator RsbQ-like family of α/β hydrolases contains the strigolactone (SL) receptor DWARF14 (AtD14), the karrikin receptor KARRIKIN INSENSITIVE2 (AtKAI2), and DWARF14-LIKE2 (AtDLK2), a protein of unknown function. Despite very similar protein folds, AtD14 and AtKAI2 differ in size and architecture of their ligand binding pockets, influencing their substrate specificity. We present the 1.5 Å crystal structure of AtDLK2, revealing the smallest ligand binding pocket in the protein family, bordered by two unique glycine residues. We identified a gatekeeper residue in the protein's lid domain and present a pyrrolo-quinoline-dione compound that inhibits AtDLK2's enzymatic activity.
© 2022 Salk Institute for Biological Studies. Plant Direct published by American Society of Plant Biologists and the Society for Experimental Biology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
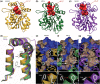


Similar articles
-
Comprehensive Analysis of DWARF14-LIKE2 (DLK2) Reveals Its Functional Divergence from Strigolactone-Related Paralogs.Front Plant Sci. 2017 Sep 22;8:1641. doi: 10.3389/fpls.2017.01641. eCollection 2017. Front Plant Sci. 2017. PMID: 28970845 Free PMC article.
-
Specialisation within the DWARF14 protein family confers distinct responses to karrikins and strigolactones in Arabidopsis.Development. 2012 Apr;139(7):1285-95. doi: 10.1242/dev.074567. Epub 2012 Feb 22. Development. 2012. PMID: 22357928
-
Strigolactone Hormones and Their Stereoisomers Signal through Two Related Receptor Proteins to Induce Different Physiological Responses in Arabidopsis.Plant Physiol. 2014 Jul;165(3):1221-1232. doi: 10.1104/pp.114.240036. Epub 2014 May 7. Plant Physiol. 2014. PMID: 24808100 Free PMC article.
-
The karrikin response system of Arabidopsis.Plant J. 2014 Aug;79(4):623-31. doi: 10.1111/tpj.12430. Epub 2014 Feb 24. Plant J. 2014. PMID: 24433542 Review.
-
Masks Start to Drop: Suppressor of MAX2 1-Like Proteins Reveal Their Many Faces.Front Plant Sci. 2022 May 12;13:887232. doi: 10.3389/fpls.2022.887232. eCollection 2022. Front Plant Sci. 2022. PMID: 35645992 Free PMC article. Review.
Cited by
-
Karrikin signalling: impacts on plant development and abiotic stress tolerance.J Exp Bot. 2024 Feb 12;75(4):1174-1186. doi: 10.1093/jxb/erad476. J Exp Bot. 2024. PMID: 38001035 Free PMC article.
-
The Multifaceted Impact of Karrikin Signaling in Plants.Int J Mol Sci. 2025 Mar 19;26(6):2775. doi: 10.3390/ijms26062775. Int J Mol Sci. 2025. PMID: 40141418 Free PMC article. Review.
References
-
- Adams, P. D. , Afonine, P. V. , Bunkoczi, G. , Chen, V. B. , Davis, I. W. , Echols, N. , Headd, J. J. , Hung, L. W. , Kapral, G. J. , Grosse‐Kunstleve, R. W. , McCoy, A. J. , Moriarty, N. W. , Oeffner, R. , Read, R. J. , Richardson, D. C. , Richardson, J. S. , Terwilliger, T. C. , & Zwart, P. H. (2010). PHENIX: A comprehensive Python‐based system for macromolecular structure solution. Acta Crystallographica. Section D, Biological Crystallography, 66, 213–221. 10.1107/S0907444909052925 - DOI - PMC - PubMed
-
- Afonine, P. V. , Grosse‐Kunstleve, R. W. , Echols, N. , Headd, J. J. , Moriarty, N. W. , Mustyakimov, M. , Terwilliger, T. C. , Urzhumtsev, A. , Zwart, P. H. , & Adams, P. D. (2012). Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallographica. Section D, Biological Crystallography, 68, 352–367. 10.1107/S0907444912001308 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

