Design, synthesis and biological evaluation of novel O-substituted tryptanthrin oxime derivatives as c-Jun N-terminal kinase inhibitors
- PMID: 36172181
- PMCID: PMC9510750
- DOI: 10.3389/fphar.2022.958687
Design, synthesis and biological evaluation of novel O-substituted tryptanthrin oxime derivatives as c-Jun N-terminal kinase inhibitors
Abstract
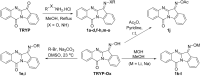
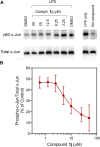
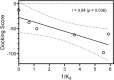
The c-Jun N-terminal kinase (JNK) family includes three proteins (JNK1-3) that regulate many physiological processes, including inflammatory responses, morphogenesis, cell proliferation, differentiation, survival, and cell death. Therefore, JNK represents an attractive target for therapeutic intervention. Herein, a panel of novel tryptanthrin oxime analogs were synthesized and evaluated for JNK1-3 binding (Kd) and inhibition of cellular inflammatory responses (IC50). Several compounds exhibited submicromolar JNK binding affinity, with the most potent inhibitor being 6-(acetoxyimino)indolo[2,1-b]quinazolin-12(6H)-one (1j), which demonstrated high JNK1-3 binding affinity (Kd = 340, 490, and 180 nM for JNK1, JNK2, and JNK3, respectively) and inhibited lipopolysaccharide (LPS)-induced nuclear factor-κB/activating protein 1 (NF-κB/AP-1) transcription activity in THP-1Blue cells and interleukin-6 (IL-6) production in MonoMac-6 monocytic cells (IC50 = 0.8 and 1.7 μM, respectively). Compound 1j also inhibited LPS-induced production of several other proinflammatory cytokines, including IL-1α, IL-1β, granulocyte-macrophage colony-stimulating factor (GM-CSF), monocyte chemoattractant protein-1 (MCP-1), and tumor necrosis factor (TNF) in MonoMac-6 cells. Likewise, 1j inhibited LPS-induced c-Jun phosphorylation in MonoMac-6 cells, directly confirming JNK inhibition. Molecular modeling suggested modes of binding interaction of selected compounds in the JNK3 catalytic site that were in agreement with the experimental JNK3 binding data. Our results demonstrate the potential for developing anti-inflammatory drugs based on these nitrogen-containing heterocyclic systems.
Keywords: 11H-indeno[1,2-b]quinoxalin-11-one; anti-inflammatory; c-Jun N-terminal kinase; interleukin-6; nuclear factor-κB; oxime; tryptanthrin.
Copyright © 2022 Schepetkin, Kovrizhina, Stankevich, Khlebnikov, Kirpotina, Quinn and Cook.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Novel Tryptanthrin Derivatives with Selectivity as c-Jun N-Terminal Kinase (JNK) 3 Inhibitors.Molecules. 2023 Jun 16;28(12):4806. doi: 10.3390/molecules28124806. Molecules. 2023. PMID: 37375361 Free PMC article.
-
Synthesis, biological evaluation, and molecular modeling of 11H-indeno[1,2-b]quinoxalin-11-one derivatives and tryptanthrin-6-oxime as c-Jun N-terminal kinase inhibitors.Eur J Med Chem. 2019 Jan 1;161:179-191. doi: 10.1016/j.ejmech.2018.10.023. Epub 2018 Oct 12. Eur J Med Chem. 2019. PMID: 30347329 Free PMC article.
-
Neuroprotective Effects of the Lithium Salt of a Novel JNK Inhibitor in an Animal Model of Cerebral Ischemia-Reperfusion.Biomedicines. 2022 Aug 29;10(9):2119. doi: 10.3390/biomedicines10092119. Biomedicines. 2022. PMID: 36140222 Free PMC article.
-
Identification and characterization of a novel class of c-Jun N-terminal kinase inhibitors.Mol Pharmacol. 2012 Jun;81(6):832-45. doi: 10.1124/mol.111.077446. Epub 2012 Mar 20. Mol Pharmacol. 2012. PMID: 22434859 Free PMC article.
-
Novel c-Jun N-Terminal Kinase (JNK) Inhibitors with an 11H-Indeno[1,2-b]quinoxalin-11-one Scaffold.Molecules. 2021 Sep 20;26(18):5688. doi: 10.3390/molecules26185688. Molecules. 2021. PMID: 34577159 Free PMC article.
Cited by
-
Identification of Potential JNK3 Inhibitors: A Combined Approach Using Molecular Docking and Deep Learning-Based Virtual Screening.Pharmaceuticals (Basel). 2023 Oct 13;16(10):1459. doi: 10.3390/ph16101459. Pharmaceuticals (Basel). 2023. PMID: 37895928 Free PMC article.
-
Experimental and Computational Investigation of the Oxime Bond Stereochemistry in c-Jun N-terminal Kinase 3 Inhibitors 11H-Indeno[1,2-b]quinoxalin-11-one Oxime and Tryptanthrin-6-oxime.Pharmaceutics. 2023 Jun 23;15(7):1802. doi: 10.3390/pharmaceutics15071802. Pharmaceutics. 2023. PMID: 37513989 Free PMC article.
-
Novel Tryptanthrin Derivatives with Selectivity as c-Jun N-Terminal Kinase (JNK) 3 Inhibitors.Molecules. 2023 Jun 16;28(12):4806. doi: 10.3390/molecules28124806. Molecules. 2023. PMID: 37375361 Free PMC article.
-
Immunomodulatory Activity of Polysaccharides Isolated from Saussurea salicifolia L. and Saussurea frolovii Ledeb.Molecules. 2023 Sep 16;28(18):6655. doi: 10.3390/molecules28186655. Molecules. 2023. PMID: 37764432 Free PMC article.
-
Tricyclic Isatin Derivatives as Anti-Inflammatory Compounds with High Kinase Binding Affinity.Molecules. 2025 Jul 10;30(14):2914. doi: 10.3390/molecules30142914. Molecules. 2025. PMID: 40733180 Free PMC article.
References
-
- Blanco F., Alkorta I., Elguero J. (2009). Barriers about double carbon-nitrogen bond in imine derivatives (aldimines, oximes, hydrazones, azines). Croat. Chem. Acta 82 (1), 173.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

