Proteomics unite traditional toxicological assessment methods to evaluate the toxicity of iron oxide nanoparticles
- PMID: 36172182
- PMCID: PMC9512491
- DOI: 10.3389/fphar.2022.1011065
Proteomics unite traditional toxicological assessment methods to evaluate the toxicity of iron oxide nanoparticles
Abstract
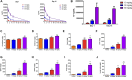
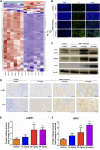
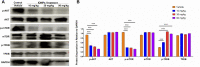
Iron oxide nanoparticles (IONPs) are the first generation of nanomaterials approved by the Food and Drug Administration for use as imaging agents and for the treatment of iron deficiency in chronic kidney disease. However, several IONPs-based imaging agents have been withdrawn because of toxic effects and the poor understanding of the underlying mechanisms. This study aimed to evaluate IONPs toxicity and to elucidate the underlying mechanism after intravenous administration in rats. Seven-week-old rats were intravenously administered IONPs at doses of 0, 10, 30, and 90 mg/kg body weight for 14 consecutive days. Toxicity and molecular perturbations were evaluated using traditional toxicological assessment methods and proteomics approaches, respectively. The administration of 90 mg/kg IONPs induced mild toxic effects, including abnormal clinical signs, lower body weight gain, changes in serum biochemical and hematological parameters, and increased organ coefficients in the spleen, liver, heart, and kidneys. Toxicokinetics, tissue distribution, histopathological, and transmission electron microscopy analyses revealed that the spleen was the primary organ for IONPs elimination from the systemic circulation and that the macrophage lysosomes were the main organelles of IONPs accumulation after intravenous administration. We identified 197 upregulated and 75 downregulated proteins in the spleen following IONPs administration by proteomics. Mechanically, the AKT/mTOR/TFEB signaling pathway facilitated autophagy and lysosomal activation in splenic macrophages. This is the first study to elucidate the mechanism of IONPs toxicity by combining proteomics with traditional methods for toxicity assessment.
Keywords: AKT/mTOR/TFEB signaling pathway; autophagy; iron oxide nanoparticles; lysosome; proteomics; toxicity.
Copyright © 2022 Han, Tian, Wang, Li, Yin, Qu, Yan, Ding, Guan and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Allard-Vannier E., Cohen-Jonathan S., Gautier J., Herve-Aubert K., Munnier E., Souce M., et al. (2012). Pegylated magnetic nanocarriers for doxorubicin delivery: A quantitative determination of stealthiness in vitro and in vivo . Eur. J. Pharm. Biopharm. 81 (3), 498–505. 10.1016/j.ejpb.2012.04.002 - DOI - PubMed
-
- Askri D., Cunin V., Beal D., Berthier S., Chovelon B., Arnaud J., et al. (2019a). Investigating the toxic effects induced by iron oxide nanoparticles on neuroblastoma cell line: An integrative study combining cytotoxic, genotoxic and proteomic tools. Nanotoxicology 13 (8), 1021–1040. 10.1080/17435390.2019.1621399 - DOI - PubMed
-
- Askri D., Cunin V., Ouni S., Beal D., Rachidi W., Sakly M., et al. (2019b). Effects of iron oxide nanoparticles (gamma-Fe2O3) on liver, lung and brain proteomes following sub-acute intranasal exposure: A new toxicological assessment in rat model using iTRAQ-based quantitative proteomics. Int. J. Mol. Sci. 20 (20), E5186. 10.3390/ijms20205186 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

