Pharmacokinetics of immediate and sustained-release formulations of paroxetine: Population pharmacokinetic approach to guide paroxetine personalized therapy in chinese psychotic patients
- PMID: 36172189
- PMCID: PMC9510632
- DOI: 10.3389/fphar.2022.966622
Pharmacokinetics of immediate and sustained-release formulations of paroxetine: Population pharmacokinetic approach to guide paroxetine personalized therapy in chinese psychotic patients
Abstract
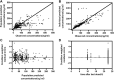
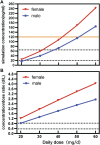
Paroxetine is one of the most potent selective serotonin reuptake inhibitors (SSRIs) approved for treating depression, panic disorder, and obsessive-compulsive disorder. There is evidence linking genetic polymorphisms and nonlinear metabolism to the Paroxetine's pharmacokinetic (PK) variability. The purpose of the present study was to develop a population PK (PPK) model of paroxetine in Chinese patients, which was used to define the paroxetine's PK parameters and quantify the effect of clinical and baseline demographic factors on these PK characteristics. The study included 184 inpatients with psychosis (103 females and 81 males), with a total of 372 serum concentrations of paroxetine for PPK analyses. The total daily dosage ranged from 20 to 75 mg. One compartment model could fit the PKs characterize of paroxetine. Covariate analysis revealed that dose, formulation, and sex had a significant effect on the PK parameters of paroxetine; however, there was no evident genetic influence of CYP2D6 enzymes on paroxetine concentrations in Chinese patients. The study determined that the population's apparent distribution volume (V/F) and apparent clearance (CL/F), respectively, were 8850 and 21.2 L/h. The CL/F decreased 1-2-fold for each 10 mg dose increase, whereas the different formulations caused a decrease in V/F of 66.6%. Sex was found to affect bioavailability (F), which decreased F by 47.5%. Females had higher F values than males. This PPK model described data from patients with psychosis who received paroxetine immediate-release tablets (IR-T) and/or sustained-release tablets (SR-T). Paroxetine trough concentrations and relative bioavailability were different between formulations and sex. The altered serum concentrations of paroxetine resulting from individual variants and additive effects need to be considered, to optimize the dosage regimen for individual patients.
Keywords: Chinese; dose-dependent; formulation; paroxetine; population pharmacokinetics.
Copyright © 2022 Li, Huang, Xiao, Wang, Kong, Liu, Zhang, Yang, Huang, Ni, Lu, Zhang, Wen and Shang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Paroxetine : a review of its pharmacology and therapeutic potential in the management of panic disorder.CNS Drugs. 1997 Aug;8(2):163-88. doi: 10.2165/00023210-199708020-00010. CNS Drugs. 1997. PMID: 23338224
-
Paroxetine: population pharmacokinetic analysis in late-life depression using sparse concentration sampling.Br J Clin Pharmacol. 2006 May;61(5):558-69. doi: 10.1111/j.1365-2125.2006.02629.x. Br J Clin Pharmacol. 2006. PMID: 16669849 Free PMC article. Clinical Trial.
-
The first study in pediatric: Population pharmacokinetics of sirolimus and its application in Chinese children with immune cytopenia.Int J Immunopathol Pharmacol. 2020 Jan-Dec;34:2058738420934936. doi: 10.1177/2058738420934936. Int J Immunopathol Pharmacol. 2020. PMID: 32720540 Free PMC article.
-
Pharmacokinetics, drug interactions and exposure-response relationship of eslicarbazepine acetate in adult patients with partial-onset seizures: population pharmacokinetic and pharmacokinetic/pharmacodynamic analyses.CNS Drugs. 2012 Jan 1;26(1):79-91. doi: 10.2165/11596290-000000000-00000. CNS Drugs. 2012. PMID: 22171585 Review.
-
Bupropion for major depressive disorder: Pharmacokinetic and formulation considerations.Clin Ther. 2005 Nov;27(11):1685-95. doi: 10.1016/j.clinthera.2005.11.011. Clin Ther. 2005. PMID: 16368442 Review.
Cited by
-
Comparative Efficacy of Animal Depression Models and Antidepressant Treatment: A Systematic Review and Meta-Analysis.Pharmaceutics. 2024 Aug 29;16(9):1144. doi: 10.3390/pharmaceutics16091144. Pharmaceutics. 2024. PMID: 39339181 Free PMC article. Review.
-
Polymyxins: recent advances and challenges.Front Pharmacol. 2024 Jun 21;15:1424765. doi: 10.3389/fphar.2024.1424765. eCollection 2024. Front Pharmacol. 2024. PMID: 38974043 Free PMC article. Review.
-
Quantifying the impacts of volume-based procurement policy on spatial accessibility of antidepressants via generic substitution: A four-city cohort study using drug sales data.PLoS One. 2025 Feb 10;20(2):e0318509. doi: 10.1371/journal.pone.0318509. eCollection 2025. PLoS One. 2025. PMID: 39928638 Free PMC article.
-
Nomograms based on clinical factors to predict abnormal metabolism of psychotropic drugs.Biomed Rep. 2025 Mar 11;22(5):83. doi: 10.3892/br.2025.1961. eCollection 2025 May. Biomed Rep. 2025. PMID: 40151799 Free PMC article.
-
Advancing paroxetine treatment in depression: predicting remission and plasma concentration, and validating and updating therapeutic reference ranges.Transl Psychiatry. 2025 Aug 28;15(1):321. doi: 10.1038/s41398-025-03503-3. Transl Psychiatry. 2025. PMID: 40877220 Free PMC article.
References
-
- DeVane C. L. (2003). Immediate-release versus controlled-release formulations: pharmacokinetics of newer antidepressants in relation to nausea. J. Clin. Psychiatry 64 (18), 14–19. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

