Stimulation of Acanthamoeba castellanii excystment by enzyme treatment and consequences on trophozoite growth
- PMID: 36172275
- PMCID: PMC9511172
- DOI: 10.3389/fcell.2022.982897
Stimulation of Acanthamoeba castellanii excystment by enzyme treatment and consequences on trophozoite growth
Abstract
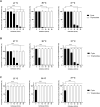
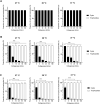
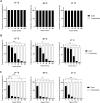
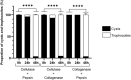
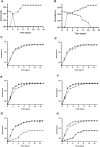
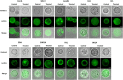
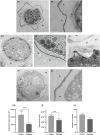
Acanthamoeba castellanii is a widespread Free-Living Amoeba (FLA) that can cause severe ocular or cerebral infections in immunocompetent and immunocompromised patients, respectively, besides its capacity to transport diverse pathogens. During their life cycle, FLA can alternate between a vegetative form, called a trophozoite, and a latent and resistant form, called a cyst. This resistant form is characterized by the presence of a cell wall containing two layers, namely the ectocyst and the endocyst, mainly composed of cellulose and proteins. In the present work, we aimed to stimulate Acanthamoeba castellanii excystment by treating their cysts with a cellulolytic enzyme, i.e., cellulase, or two proteolytic enzymes, i.e., collagenase and pepsin. While 11 days were necessary to obtain total excystment in the control at 27°C, only 48 h were sufficient at the same temperature to obtain 100% trophozoites in the presence of 25 U/mL cellulase, 50 U/mL collagenase or 100 U/mL pepsin. Additionally, more than 96% amoebae have excysted after only 24 h with 7.5 U/mL cellulase at 30°C. Nevertheless, no effect of the three enzymes was observed on the excystment of Balamuthia mandrillaris and Vermamoeba vermiformis. Surprisingly, A. castellanii trophozoites excysted in the presence of cellulase displayed a markedly shorter doubling time at 7 h, in comparison to the control at 23 h. Likewise, trophozoites doubled their population in 9 h when both cellulose and cellulase were added to the medium, indicating that Acanthamoeba cyst wall degradation products promote their trophozoite proliferation. The analysis of cysts in epifluorescent microscopy using FITC-lectins and in electron microscopy revealed a disorganized endocyst and a reduction of the intercystic space area after cellulase treatment, implying that these cellular events are preliminary to trophozoite release during excystment. Further studies would be necessary to determine the signaling pathways involved during this amoebal differentiation process to identify new therapeutic targets for the development of anti-acanthamoebal drugs.
Keywords: Acanthamoeba; cellulase; excystment; free-living amoeba; protease; trophozoite growth.
Copyright © 2022 Fechtali-Moute, Loiseau and Pomel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Encystment and Excystment Processes in Acanthamoeba castellanii: An Emphasis on Cellulose Involvement.Pathogens. 2025 Mar 10;14(3):268. doi: 10.3390/pathogens14030268. Pathogens. 2025. PMID: 40137753 Free PMC article. Review.
-
An Alternative Procedure for the Storage and Preservation of Vermamoeba vermiformis and Other Amoebae in the Laboratory.Curr Protoc. 2025 Jun;5(6):e70164. doi: 10.1002/cpz1.70164. Curr Protoc. 2025. PMID: 40552954
-
Morphological Study of the Encystment and Excystment of Vermamoeba vermiformis Revealed Original Traits.J Eukaryot Microbiol. 2015 May-Jun;62(3):327-37. doi: 10.1111/jeu.12185. Epub 2014 Oct 29. J Eukaryot Microbiol. 2015. PMID: 25284205
-
Acanthamoeba castellanii: growth, encystment, excystment and biocide susceptibility.J Infect. 1998 Jan;36(1):43-8. doi: 10.1016/s0163-4453(98)93054-7. J Infect. 1998. PMID: 9515667
-
Cellulose degradation: a therapeutic strategy in the improved treatment of Acanthamoeba infections.Parasit Vectors. 2015 Jan 14;8:23. doi: 10.1186/s13071-015-0642-7. Parasit Vectors. 2015. PMID: 25586209 Free PMC article. Review.
Cited by
-
Influence of the Age of Free-Living Amoeba Cysts on Their Vertical Distribution in a Water Column.Microorganisms. 2024 Feb 27;12(3):474. doi: 10.3390/microorganisms12030474. Microorganisms. 2024. PMID: 38543525 Free PMC article.
-
The in vitro effect riboflavin combined with or without UVA in Acanthamoeba castellanii.Sci Rep. 2024 Nov 5;14(1):26799. doi: 10.1038/s41598-024-77787-8. Sci Rep. 2024. PMID: 39500946 Free PMC article.
-
Encystment and Excystment Processes in Acanthamoeba castellanii: An Emphasis on Cellulose Involvement.Pathogens. 2025 Mar 10;14(3):268. doi: 10.3390/pathogens14030268. Pathogens. 2025. PMID: 40137753 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

