Intranasal administration of a single dose of MVA-based vaccine candidates against COVID-19 induced local and systemic immune responses and protects mice from a lethal SARS-CoV-2 infection
- PMID: 36172368
- PMCID: PMC9510595
- DOI: 10.3389/fimmu.2022.995235
Intranasal administration of a single dose of MVA-based vaccine candidates against COVID-19 induced local and systemic immune responses and protects mice from a lethal SARS-CoV-2 infection
Abstract
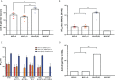
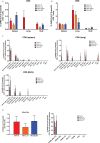
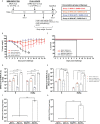
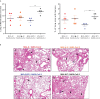
Current coronavirus disease-19 (COVID-19) vaccines are administered by the intramuscular route, but this vaccine administration failed to prevent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus infection in the upper respiratory tract, mainly due to the absence of virus-specific mucosal immune responses. It is hypothesized that intranasal (IN) vaccination could induce both mucosal and systemic immune responses that blocked SARS-CoV-2 transmission and COVID-19 progression. Here, we evaluated in mice IN administration of three modified vaccinia virus Ankara (MVA)-based vaccine candidates expressing the SARS-CoV-2 spike (S) protein, either the full-length native S or a prefusion-stabilized [S(3P)] protein; SARS-CoV-2-specific immune responses and efficacy were determined after a single IN vaccine application. Results showed that in C57BL/6 mice, MVA-based vaccine candidates elicited S-specific IgG and IgA antibodies in serum and bronchoalveolar lavages, respectively, and neutralizing antibodies against parental and SARS-CoV-2 variants of concern (VoC), with MVA-S(3P) being the most immunogenic vaccine candidate. IN vaccine administration also induced polyfunctional S-specific Th1-skewed CD4+ and cytotoxic CD8+ T-cell immune responses locally (in lungs and bronchoalveolar lymph nodes) or systemically (in spleen). Remarkably, a single IN vaccine dose protected susceptible K18-hACE2 transgenic mice from morbidity and mortality caused by SARS-CoV-2 infection, with MVA-S(3P) being the most effective candidate. Infectious SARS-CoV-2 viruses were undetectable in lungs and nasal washes, correlating with high titers of S-specific IgGs and neutralizing antibodies against parental SARS-CoV-2 and several VoC. Moreover, low histopathological lung lesions and low levels of pro-inflammatory cytokines in lungs and nasal washes were detected in vaccinated animals. These results demonstrated that a single IN inoculation of our MVA-based vaccine candidates induced potent immune responses, either locally or systemically, and protected animal models from COVID-19. These results also identified an effective vaccine administration route to induce mucosal immunity that should prevent SARS-CoV-2 host-to-host transmission.
Keywords: MVA; S protein; SARS-CoV-2; immunogenicity; intranasal delivery; mice; protective efficacy; vaccine candidates.
Copyright © 2022 Pérez, Astorgano, Albericio, Flores, Sánchez-Cordón, Luczkowiak, Delgado, Casasnovas, Esteban and García-Arriaza.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
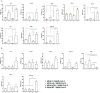
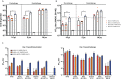
Similar articles
-
MVA-based vaccine candidates expressing SARS-CoV-2 prefusion-stabilized spike proteins of the Wuhan, Beta or Omicron BA.1 variants protect transgenic K18-hACE2 mice against Omicron infection and elicit robust and broad specific humoral and cellular immune responses.Front Immunol. 2024 Aug 29;15:1420304. doi: 10.3389/fimmu.2024.1420304. eCollection 2024. Front Immunol. 2024. PMID: 39267752 Free PMC article.
-
A Single Dose of an MVA Vaccine Expressing a Prefusion-Stabilized SARS-CoV-2 Spike Protein Neutralizes Variants of Concern and Protects Mice From a Lethal SARS-CoV-2 Infection.Front Immunol. 2022 Jan 27;12:824728. doi: 10.3389/fimmu.2021.824728. eCollection 2021. Front Immunol. 2022. PMID: 35154086 Free PMC article.
-
Preclinical immune efficacy against SARS-CoV-2 beta B.1.351 variant by MVA-based vaccine candidates.Front Immunol. 2023 Dec 12;14:1264323. doi: 10.3389/fimmu.2023.1264323. eCollection 2023. Front Immunol. 2023. PMID: 38155964 Free PMC article.
-
Intranasal COVID-19 vaccines: From bench to bed.EBioMedicine. 2022 Feb;76:103841. doi: 10.1016/j.ebiom.2022.103841. Epub 2022 Jan 24. EBioMedicine. 2022. PMID: 35085851 Free PMC article. Review.
-
Studies of SARS virus vaccines.Hong Kong Med J. 2008 Aug;14 Suppl 4:39-43. Hong Kong Med J. 2008. PMID: 18708674 Review.
Cited by
-
A multi-antigen vaccinia vaccine broadly protected mice against SARS-CoV-2 and influenza A virus while also targeting SARS-CoV-1 and MERS-CoV.Front Immunol. 2024 Nov 28;15:1473428. doi: 10.3389/fimmu.2024.1473428. eCollection 2024. Front Immunol. 2024. PMID: 39669563 Free PMC article.
-
The role of vaccination route with an adenovirus-vectored vaccine in protection, viral control, and transmission in the SARS-CoV-2/K18-hACE2 mouse infection model.Front Immunol. 2023 Aug 16;14:1188392. doi: 10.3389/fimmu.2023.1188392. eCollection 2023. Front Immunol. 2023. PMID: 37662899 Free PMC article.
-
Edible Plant-Derived Extracellular Vesicles for Oral mRNA Vaccine Delivery.Vaccines (Basel). 2024 Feb 15;12(2):200. doi: 10.3390/vaccines12020200. Vaccines (Basel). 2024. PMID: 38400183 Free PMC article. Review.
-
Optimized vaccine candidate MVA-S(3P) fully protects against SARS-CoV-2 infection in hamsters.Front Immunol. 2023 Oct 18;14:1163159. doi: 10.3389/fimmu.2023.1163159. eCollection 2023. Front Immunol. 2023. PMID: 37920464 Free PMC article.
-
Recent developments in the immunopathology of COVID-19.Allergy. 2023 Feb;78(2):369-388. doi: 10.1111/all.15593. Epub 2022 Dec 5. Allergy. 2023. PMID: 36420736 Free PMC article. Review.
References
-
- Bricker TL, Darling TL, Hassan AO, Harastani HH, Soung A, Jiang X, et al. . A single intranasal or intramuscular immunization with chimpanzee adenovirus-vectored SARS-CoV-2 vaccine protects against pneumonia in hamsters. Cell Rep (2021) 36(3):109400. doi: 10.1016/j.celrep.2021.109400 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

