Role of RGC-32 in multiple sclerosis and neuroinflammation - few answers and many questions
- PMID: 36172382
- PMCID: PMC9510783
- DOI: 10.3389/fimmu.2022.979414
Role of RGC-32 in multiple sclerosis and neuroinflammation - few answers and many questions
Abstract
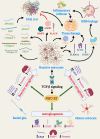
Recent advances in understanding the pathogenesis of multiple sclerosis (MS) have brought into the spotlight the major role played by reactive astrocytes in this condition. Response Gene to Complement (RGC)-32 is a gene induced by complement activation, growth factors, and cytokines, notably transforming growth factor β, that is involved in the modulation of processes such as angiogenesis, fibrosis, cell migration, and cell differentiation. Studies have uncovered the crucial role that RGC-32 plays in promoting the differentiation of Th17 cells, a subtype of CD4+ T lymphocytes with an important role in MS and its murine model, experimental autoimmune encephalomyelitis. The latest data have also shown that RGC-32 is involved in regulating major transcriptomic changes in astrocytes and in favoring the synthesis and secretion of extracellular matrix components, growth factors, axonal growth molecules, and pro-astrogliogenic molecules. These results suggest that RGC-32 plays a major role in driving reactive astrocytosis and the generation of astrocytes from radial glia precursors. In this review, we summarize recent advances in understanding how RGC-32 regulates the behavior of Th17 cells and astrocytes in neuroinflammation, providing insight into its role as a potential new biomarker and therapeutic target.
Keywords: EAE (experimental autoimmune encephalomyelitis); RGC-32; Th17; astrocyte; multiple sclerosis; neuroinflammation; radial glia.
Copyright © 2022 Tatomir, Cuevas, Badea, Muresanu, Rus and Rus.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

