Exploring wild Aspleniaceae ferns as safety sources of polyphenols: The case of Asplenium trichomanes L. and Ceterach officinarum Willd
- PMID: 36172521
- PMCID: PMC9511145
- DOI: 10.3389/fnut.2022.994215
Exploring wild Aspleniaceae ferns as safety sources of polyphenols: The case of Asplenium trichomanes L. and Ceterach officinarum Willd
Abstract
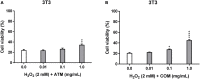
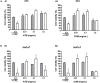
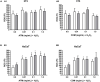
The forest ecosystem is a source of material resources used since ancient times by mankind. Ferns are part of different oriental systems of traditional medicine due to the phytochemical variety of their fronds, which have allowed their traditional use to be validated through ethnopharmacological studies. In Europe, different cultures have used the same fern with a wide variety of applications due to its presence in most European forests. In recent years, studies on the phytocharacterization and biological activity of the fronds of the main European ferns have been published. In this study, the presence of polyphenolic phytochemicals has been evaluated by high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) in the fronds of two wild ferns together with in vitro activities in non-tumoral and human tumoral cell lines. The polyphenols were extracted from Asplenium trichomanes L. and Ceterach officinarum Willd. by cold maceration using methanol. The main phytochemicals of polyphenolic origin in the extracts of A. trichomanes and C. officinarum determined by HPLC-MS/MS were the flavonol hyperoside and the phenolic acid chlorogenic acid, respectively. This different polyphenolic nature of both extracts contributes to the divergence of the behavior experienced in the biological activities tested, but none of the extracts showed a cytotoxic or phototoxic profile in the different tested cell lines. However, the cytoprotective values in front of the H2O2 oxidative stress induced in the 3T3 and HaCaT cell lines position these extracts as possible candidates for future health applications.
Keywords: bioeconomy; cytoprotection; cytotoxicty; ethnopharmacology; ferns; functional food; polyphenolic phytochemicals.
Copyright © 2022 Farràs, Mitjans, Maggi, Caprioli, Vinardell and López.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Polypodium vulgare L. (Polypodiaceae) as a Source of Bioactive Compounds: Polyphenolic Profile, Cytotoxicity and Cytoprotective Properties in Different Cell Lines.Front Pharmacol. 2021 Sep 16;12:727528. doi: 10.3389/fphar.2021.727528. eCollection 2021. Front Pharmacol. 2021. PMID: 34603041 Free PMC article.
-
Selective Anticancer Properties, Proapoptotic and Antibacterial Potential of Three Asplenium Species.Plants (Basel). 2021 May 25;10(6):1053. doi: 10.3390/plants10061053. Plants (Basel). 2021. PMID: 34070269 Free PMC article.
-
Essential Oil Constituents and Antioxidant Activity of Asplenium Ferns.J Chromatogr Sci. 2016 Sep;54(8):1341-5. doi: 10.1093/chromsci/bmw071. Epub 2016 May 10. J Chromatogr Sci. 2016. PMID: 27165574
-
Traditional Use, Phytochemical Profiles and Pharmacological Properties of Artemisia Genus from Central Asia.Molecules. 2022 Aug 11;27(16):5128. doi: 10.3390/molecules27165128. Molecules. 2022. PMID: 36014364 Free PMC article. Review.
-
Ethnomedical, phytochemical and pharmacological insights on an Indian medicinal plant: The balloon vine (Cardiospermum halicacabum Linn.).J Ethnopharmacol. 2022 Jun 12;291:115143. doi: 10.1016/j.jep.2022.115143. Epub 2022 Feb 25. J Ethnopharmacol. 2022. PMID: 35227784 Review.
Cited by
-
Phytochemical profile and antimicrobial activity of individual frond extracts of Menisorus pauciflorus, Pteris catoptera, Conniogramme africana and Antrophyum mannianum.BMC Complement Med Ther. 2025 Jul 12;25(1):262. doi: 10.1186/s12906-025-05003-9. BMC Complement Med Ther. 2025. PMID: 40652226 Free PMC article.
-
Kidney Stone Prevention: Is There a Role for Complementary and Alternative Medicine?Nutrients. 2023 Feb 9;15(4):877. doi: 10.3390/nu15040877. Nutrients. 2023. PMID: 36839235 Free PMC article. Review.
References
-
- Chamberlain JL, Darr D, Meinhold K. Rediscovering the contributions of forests and trees to transition global food systems. Forests. (2020) 11:21. 10.3390/f11101098 - DOI
-
- Jankovsky M, García-Jácome SP, Dvorák J, Nyarko I, Hájek M. Innovations in forest bioeconomy: A bibliometric analysis. Forests. (2021) 12:17. 10.3390/f12101392 - DOI
-
- Haines A. Health in the bioeconomy. Lancet Planet Health. (2021) 5:E4–5. - PubMed
-
- Nagalingum NS. Seedless land plants, evolution and diversification of. In: Kliman RM. editor. Encyclopedia of Evolutionary Biology (Sydney, NSW: Elsevier; ) (2016). 10.1016/B978-0-12-800049-6.00255-9 - DOI
LinkOut - more resources
Full Text Sources

