In vivo activity and atom pair fingerprint analysis of MMV665941 against the apicomplexan parasite Babesia microti, the causative agent of babesiosis in humans and rodents
- PMID: 36172647
- PMCID: PMC10081058
- DOI: 10.1080/20477724.2022.2128571
In vivo activity and atom pair fingerprint analysis of MMV665941 against the apicomplexan parasite Babesia microti, the causative agent of babesiosis in humans and rodents
Abstract
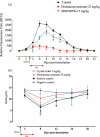
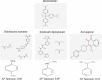
The effect of MMV665941 on the growth of Babesia microti (B. microti) in mice, was investigated in this study using a fluorescence-based SYBR Green I test. Using atom Pair signatures, we investigated the structural similarity between MMV665941 and the commonly used antibabesial medicines diminazene aceturate (DA), imidocarb dipropionate (ID), or atovaquone (AV). In vitro cultures of Babesia bovis (B. bovis) and, Theileria equi (T. equi) were utilized to determine the MMV665941 and AV interaction using combination ratios ranged from 0.75 IC50 MMV665941:0.75 IC50 AV to 0.50 IC50 MMV665941:0.50 IC50 AV. The used combinations were prepared depending on the IC50 of each drug against the in vitro growth of the tested parasite. Every 96 h, the hemolytic anemia in the treated mice was monitored using a Celltac MEK-6450 computerized hematology analyzer. A single dose of 5 mg/kg MMV665941 exhibited inhibition in the B. microti growth from day 4 post-inoculation (p.i.) till day 12 p.i. MMV665941 caused 62.10%, 49.88%, and 74.23% inhibitions in parasite growth at days 4, 6 and 8 p.i., respectively. Of note, 5 mg/kg MMV665941 resulted in quick recovery of hemolytic anemia caused by babesiosis. The atom pair fingerprint (APfp) analysis revealed that MMV665941 and atovaquone (AV) showed maximum structural similarity. Of note, high concentrations (0.75 IC50) of MMV665941 and AV caused synergistic inhibition on B. bovis growth. These findings suggest that MMV665941 might be a promising drug for babesiosis treatment, particularly when combined with the commonly used antibabesial drug, AV.
Keywords: Babesia microti; MMV665941; atovaquone; in vivo; structural similarity measurements.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
Similar articles
-
Ascorbic acid co-administration with a low dose of diminazene aceturate inhibits the in vitro growth of Theileria equi, and the in vivo growth of Babesia microti.Parasitol Int. 2022 Oct;90:102596. doi: 10.1016/j.parint.2022.102596. Epub 2022 May 6. Parasitol Int. 2022. PMID: 35533961
-
Diminazene aceturate and imidocarb dipropionate-based combination therapy for babesiosis - A new paradigm.Ticks Tick Borne Dis. 2023 Jul;14(4):102145. doi: 10.1016/j.ttbdis.2023.102145. Epub 2023 Apr 1. Ticks Tick Borne Dis. 2023. PMID: 37011497
-
17-DMAG inhibits the multiplication of several Babesia species and Theileria equi on in vitro cultures, and Babesia microti in mice.Int J Parasitol Drugs Drug Resist. 2018 Apr;8(1):104-111. doi: 10.1016/j.ijpddr.2018.02.005. Epub 2018 Mar 1. Int J Parasitol Drugs Drug Resist. 2018. PMID: 29499568 Free PMC article.
-
Comparative Bioinformatics Analysis of Transcription Factor Genes Indicates Conservation of Key Regulatory Domains among Babesia bovis, Babesia microti, and Theileria equi.PLoS Negl Trop Dis. 2016 Nov 10;10(11):e0004983. doi: 10.1371/journal.pntd.0004983. eCollection 2016 Nov. PLoS Negl Trop Dis. 2016. PMID: 27832060 Free PMC article. Review.
-
Chemotherapy against babesiosis.Vet Parasitol. 2006 May 31;138(1-2):147-60. doi: 10.1016/j.vetpar.2006.01.048. Epub 2006 Feb 28. Vet Parasitol. 2006. PMID: 16504402 Review.
Cited by
-
Evaluating Antimalarial Proteasome Inhibitors for Efficacy in Babesia Blood Stage Cultures.ACS Omega. 2024 Oct 28;9(45):44989-44999. doi: 10.1021/acsomega.4c04564. eCollection 2024 Nov 12. ACS Omega. 2024. PMID: 39554424 Free PMC article.
References
-
- Rizk MA, El-Sayed SAE, El-Khodery S, et al. Discovering the in vitro potent inhibitors against Babesia and Theileria parasites by repurposing the Malaria Box: a review. Vet Parasitol. 2019;274:108895. - PubMed
-
- Rizk MA, El-Sayed SAE, Nassif M, et al. Assay methods for in vitro and in vivo anti-Babesia drug efficacy testing: current progress, outlook, and challenges. Vet Parasitol. 2020;279:109013. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
