Efficacy of a Hybrid Closed-Loop Solution in Patients With Excessive Time in Hypoglycaemia: A Post Hoc Analysis of Trials With DBLG1 System
- PMID: 36172702
- PMCID: PMC10973855
- DOI: 10.1177/19322968221128565
Efficacy of a Hybrid Closed-Loop Solution in Patients With Excessive Time in Hypoglycaemia: A Post Hoc Analysis of Trials With DBLG1 System
Abstract
Background: Automated insulin delivery is an efficient treatment for patients with type 1 diabetes. Little is known on its impact on patients with excessive time in hypoglycaemia.
Methods: We performed a post hoc analysis of three randomized control trials that used the DBLG1 (Diabeloop Generation 1) hybrid closed-loop solution. Patients whose time below 70 mg/dL during baseline, open-loop phase exceeded 5% were selected. The outcomes were the differences between the closed-loop and the open-loop phases in time in various ranges and Glycemia Risk Index (GRI).
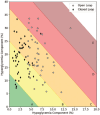
Results: We identified 45 patients exhibiting ≥5% of time below 70 mg/dL during the open-loop phase. Under closed-loop, the time in hypoglycaemia (54 to <70 mg/dL) dropped from 7.9% (SD 2.4) to 3.2% (SD 1.6) (difference -4.7% [-5.3; -4.1], P < 10-4). The time below 54 mg/dL decreased from 1.9% (SD 1.3) to 0.8% (SD 0.7) (difference -0.9% [-1.4; -0.8], P < 10-4). The time in range (TIR 70-180 mg/dL) improved from 63.3 (SD 9.5) to 68.2% (SD 8.2) (difference 5.1% [2.9; 7.0], P < 10-4). The GRI improved from 51.2 (SD 12.4) to 38.0 (SD 10.9) (difference 13.2 [10.4; 16.0], P < 10-4).
Conclusion: DBLG1 decreased time in hypoglycaemia by more than 50% even in patients with excessive time in hypoglycaemia at baseline, while also improving both TIR and GRI, under real-life conditions. The improvement in GRI (13.2%) exceeded that of the improvement in TIR (5.1%) indicating that in this data set, GRI was more sensitive than TIR to the improvement in glycaemia achieved with closed-loop. These results support the safety and efficacy of this treatment.
Trial registration: ClinicalTrials.gov NCT03671915 NCT04190277, NCT02987556.
Keywords: artificial pancreas; closed-loop; hypoglycaemia; type 1 diabetes.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: This study was funded by the French Innovation Fund (Banque Publique d’Investissement, Maisons-Alfort; France) and by Diabeloop SA (Grenoble, France). PYB, SF, and GC are consultants for Diabeloop SA. AA, YT, SP, and SM are employees from Diabeloop SA. DK is a consultant to EOFlow, Fractyl, Integrity, Lifecare, Roche Diagnostics, Rockley Photonics, and Thirdwayv. No author has been paid to write this article, and the findings and conclusions in this study are those of the authors and do not necessarily represent the views of the sponsors. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Figures

References
-
- Benhamou PY, Franc S, Reznik Y, et al. Closed-loop insulin delivery in adults with type 1 diabetes in real-life conditions: a 12-week multicentre, open-label randomised controlled crossover trial. Lancet Digit Health. 2019;1(1):e17-e25. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

